Chiral Bis(tetrathiafulvalene)-1,2-cyclohexane-diamides
- PMID: 36296517
- PMCID: PMC9611696
- DOI: 10.3390/molecules27206926
Chiral Bis(tetrathiafulvalene)-1,2-cyclohexane-diamides
Abstract
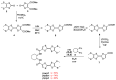
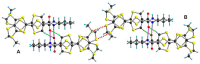
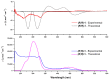
Chiral bis(TTF) diamides have been obtained in good yields (54-74%) from 1,2-cyclohexane-diamine and the corresponding TTF acyl chlorides. The (R,R)-1 and (S,S)-1 enantiomers have been characterized by circular dichroism and the racemic form by single-crystal X-ray diffraction. The neutral racemic bis(TTF)-diamide shows the formation of a pincer-like framework in the solid state, thanks to the intramolecular S···S interactions. The chemical oxidation in a solution using FeCl3 provides stable oxidized species, while the electrocrystallization experiments provided radical cation salts. In particular, single-crystal resistivity measurements on the racemic donor with AsF6- as a counterion demonstrate semiconductor behavior in this material. The DFT and TD-DFT calculations support the structural and chiroptical features of these new chiral TTF donors.
Keywords: DFT calculations; chirality; chiroptical properties; crystal structure determination; magnetic properties; tetrathiafulvalene.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures











References
-
- Avarvari N., Wallis J.D. Strategies towards Chiral Molecular Conductors. J. Mater. Chem. 2009;19:4061–4076. doi: 10.1039/b820598a. - DOI
-
- Wallis J.D., Karrer A., Dunitz J.D. Chiral metals? A chiral substrate for organic conductors and superconductors. Helv. Chim. Acta. 1986;69:69–70. doi: 10.1002/hlca.19860690110. - DOI
-
- Karrer A., Wallis J.D., Dunitz J.D., Hilti B., Mayer C.W., Bürkle M., Pfeiffer J. Structures and Electrical Properties of Some New Organic Conductors Derived from the Donor Molecule TMET (S,S,S,S-Bis(dimethylethylenedithio) tetrathiafulvalene) Helv. Chim. Acta. 1987;70:942–953. doi: 10.1002/hlca.19870700405. - DOI
-
- Krstić V., Roth S., Burghard M., Kern K., Rikken G.L.J.A. Magneto-Chiral Anisotropy in Charge Transport Through Single-Walled Carbon Nanotubes. J. Chem. Phys. 2002;117:11315–11319. doi: 10.1063/1.1523895. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

