Domino Aza-Michael-SNAr-Heteroaromatization Route to C5-Substituted 1-Alkyl-1 H-Indole-3-Carboxylic Esters
- PMID: 36296590
- PMCID: PMC9611145
- DOI: 10.3390/molecules27206998
Domino Aza-Michael-SNAr-Heteroaromatization Route to C5-Substituted 1-Alkyl-1 H-Indole-3-Carboxylic Esters
Abstract
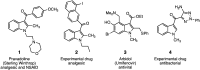
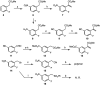
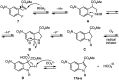
A new synthesis of C5-substituted 1-alkyl-1H-indole-3-carboxylic esters is reported. A series of methyl 2-arylacrylate aza-Michael acceptors were prepared with aromatic substitution to activate them towards SNAr reaction. Subsequent reaction with a series of primary amines generated the title compounds. Initially, the sequence was expected to produce indoline products, but oxidative heteroaromatization intervened to generate the indoles. The reaction proceeded under anhydrous conditions in DMF at 23-90 °C using equimolar quantities of the acrylate and the amine with 2 equiv. of K2CO3 to give 61-92% of the indole products. The reaction involves an aza-Michael addition, followed by SNAr ring closure and heteroaromatization. Since the reactions were run under nitrogen, the final oxidation to the indole likely results from reaction with dissolved oxygen in the DMF. Substrates incorporating a 2-arylacrylonitrile proved too reactive to prepare using our protocol. The synthesis of the reaction substrates, their relative reactivities, and mechanistic details of the conversion are discussed.
Keywords: 1H-indole synthesis; SNAr reaction; aza–Michael reaction; domino reaction; heteroaromatization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Fleming I., Woolias M. A benzyne route to indoles from o- or m-bromoaryl ketones. J. Chem. Soc. Perkin Trans. 1. 1979:827–828. doi: 10.1039/p19790000827. - DOI
-
- Ito Y., Kobayashi K., Saegusa T. An efficient synthesis of indole. J. Am. Chem. Soc. 1977;99:3532–3534. doi: 10.1021/ja00452a073. - DOI
-
- Scriven E.F.V., Turnbull K. Azides: Their preparation and synthetic uses. Chem. Rev. 1988;88:297–368. doi: 10.1021/cr00084a001. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

