Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants
- PMID: 36296686
- PMCID: PMC9610647
- DOI: 10.3390/molecules27207092
Acetylcholinesterase Inhibitory Activities of Essential Oils from Vietnamese Traditional Medicinal Plants
Abstract
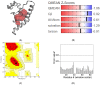
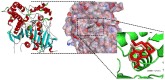
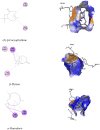
Essential oils are promising as environmentally friendly and safe sources of pesticides for human use. Furthermore, they are also of interest as aromatherapeutic agents in the treatment of Alzheimer's disease, and inhibition of the enzyme acetylcholinesterase (AChE) has been evaluated as an important mechanism. The essential oils of some species in the genera Callicarpa, Premna, Vitex and Karomia of the family Lamiaceae were evaluated for inhibition of electric eel AChE using the Ellman method. The essential oils of Callicarpa candicans showed promising activity, with IC50 values between 45.67 and 58.38 μg/mL. The essential oils of Callicarpa sinuata, Callicarpa petelotii, Callicarpa nudiflora, Callicarpa erioclona and Vitex ajugifolia showed good activity with IC50 values between 28.71 and 54.69 μg/mL. The essential oils Vitex trifolia subsp. trifolia and Callicarpa rubella showed modest activity, with IC50 values of 81.34 and 89.38, respectively. trans-Carveol showed an IC50 value of 102.88 µg/mL. Molecular docking and molecular dynamics simulation were performed on the major components of the studied essential oils to investigate the possible mechanisms of action of potential inhibitors. The results obtained suggest that these essential oils may be used to control mosquito vectors that transmit pathogenic viruses or to support the treatment of Alzheimer's disease.
Keywords: Alzheimer’s; Lamiaceae; atractylone; pesticide; trans-carveol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

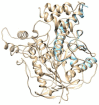


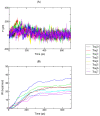
References
-
- Trang A., Khandhar P.B. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. [(accessed on 4 June 2022)]. Physiology, Acetylcholinesterase. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539735/ - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

