Oxygen Vacancies in Bismuth Tantalum Oxide to Anchor Polysulfide and Accelerate the Sulfur Evolution Reaction in Lithium-Sulfur Batteries
- PMID: 36296742
- PMCID: PMC9607072
- DOI: 10.3390/nano12203551
Oxygen Vacancies in Bismuth Tantalum Oxide to Anchor Polysulfide and Accelerate the Sulfur Evolution Reaction in Lithium-Sulfur Batteries
Abstract
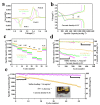
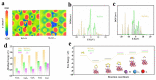
The shuttling effect of soluble lithium polysulfides (LiPSs) and the sluggish conversion kinetics of polysulfides into insoluble Li2S2/Li2S severely hinders the practical application of Li-S batteries. Advanced catalysts can capture and accelerate the liquid-solid conversion of polysulfides. Herein, we try to make use of bismuth tantalum oxide with oxygen vacancies as an electrocatalyst to catalyze the conversion of LiPSs by reducing the sulfur reduction reaction (SRR) nucleation energy barrier. Oxygen vacancies in Bi4TaO7 nanoparticles alter the electron band structure to improve instinct electronic conductivity and catalytic activity. In addition, the defective surface could provide unsaturated bonds around the vacancies to enhance the chemisorption capability with LiPSs. Hence, a multidimensional carbon (super P/CNT/Graphene) standing sulfur cathode is prepared by coating oxygen vacancies Bi4TaO7-x nanoparticles, in which the multidimensional carbon (MC) with micropores structure can host sulfur and provide a fast electron/ion pathway, while the outer-coated oxygen vacancies with Bi4TaO7-x with improved electronic conductivity and strong affinities for polysulfides can work as an adsorptive and conductive protective layer to achieve the physical restriction and chemical immobilization of lithium polysulfides as well as speed up their catalytic conversion. Benefiting from the synergistic effects of different components, the S/C@Bi3TaO7-x coin cell cathode shows superior cycling and rate performance. Even under a high level of sulfur loading of 9.6 mg cm-2, a relatively high initial areal capacity of 10.20 mAh cm-2 and a specific energy density of 300 Wh kg-1 are achieved with a low electrolyte/sulfur ratio of 3.3 µL mg-1. Combined with experimental results and theoretical calculations, the mechanism by which the Bi4TaO7 with oxygen vacancies promotes the kinetics of polysulfide conversion reactions has been revealed. The design of the multiple confined cathode structure provides physical and chemical adsorption, fast charge transfer, and catalytic conversion for polysulfides.
Keywords: electrochemical performance; high areal mass loading; lithium–sulfur battery; oxygen vacancies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Dai C.L., Hu L.Y., Wang M.Q., Chen Y.M., Han J., Jiang J., Zhang Y., Shen B., Niu Y.B., Bao S.J., et al. Uniform α-Ni(OH)2 hollow spheres constructed from ultrathin nanosheets as efficient polysulfide mediator for long-term lithium-sulfur batteries. Energy Storage Mater. 2017;8:202–208. doi: 10.1016/j.ensm.2017.04.003. - DOI
-
- Liu X., Wang S., Wang A.L., Wang Z.N., Chen J., Zeng Q.H., Chen P.P., Liu W., Li Z.X., Zhang L.Y. A new cathode material synthesized by a thiol-modified metal–organic framework (MOF) covalently connecting sulfur for superior long-cycling stability in lithium–sulfur batteries. J. Mater. Chem. A. 2019;7:24515–24523. doi: 10.1039/C9TA08043K. - DOI
-
- Ren J., Song Z.C., Zhou X.M., Chai Y.R., Lu X.L., Zheng Q.J., Xu C.G., Lin D.M. A Porous Carbon Polyhedron/Carbon Nanotube Based Hybrid Material as Multifunctional Sulfur Host for High-Performance Lithium-Sulfur Batteries. ChemElectroChem. 2019;6:3410–3419. doi: 10.1002/celc.201900744. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

