Different Strategies to Attenuate the Toxic Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells
- PMID: 36296751
- PMCID: PMC9607034
- DOI: 10.3390/nano12203561
Different Strategies to Attenuate the Toxic Effects of Zinc Oxide Nanoparticles on Spermatogonia Cells
Abstract
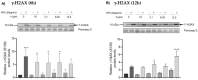
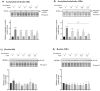
Zinc oxide nanoparticles (ZnO NPs) are one of the most used nanoparticles due to their unique physicochemical and biological properties. There is, however, a growing concern about their negative impact on male reproductive health. Therefore, in the present study, two different strategies were used to evaluate the recovery ability of spermatogonia cells from the first stage of spermatogenesis (GC-1 spg cell line) after being exposed to a cytotoxic concentration of ZnO NPs (20 µg/mL) for two different short time periods, 6 and 12 h. The first strategy was to let the GC-1 cells recover after ZnO NPs exposure in a ZnO NPs-free medium for 4 days. At this phase, cell viability assays were performed to evaluate whether this period was long enough to allow for cell recovery. Exposure to ZnO NPs for 6 h and 12 h induced a decrease in viability of 25% and 41%, respectively. However, the recovery period allowed for an increase in cell viability from 16% to 25% to values as high as 91% and 84%. These results strongly suggest that GC-1 cells recover, but not completely, given that the cell viability does not reach 100%. Additionally, the impact of a synthetic chalcone (E)-3-(2,6-dichlorophenyl)-1-(2-hydroxyphenyl)prop-2-en-1-one (1) to counteract the reproductive toxicity of ZnO NPs was investigated. Different concentrations of chalcone 1 (0-12.5 µM) were used before and during exposure of GC-1 cells to ZnO NPs to mitigate the damage induced by NPs. The protective ability of this compound was evaluated through viability assays, levels of DNA damage, and cytoskeleton dynamics (evaluating the acetylated α-tubulin and β-actin protein levels). The results indicated that the tested concentrations of chalcone 1 can attenuate the genotoxicity induced by ZnO NPs for shorter exposure periods (6 h). Chalcone 1 supplementation also increased cell viability and stabilized the microtubules. However, the antioxidant potential of this compound remains to be elucidated. In conclusion, this work addressed the main cytotoxic effects of ZnO NPs on a spermatogonia cell line and analyzed two different strategies to mitigate this damage, which represent a significant contribution to the field of male fertility.
Keywords: DNA damage; antioxidant; chalcone; cytoskeleton; cytotoxicity; male infertility; oxidative stress; reversibility; zinc oxide nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

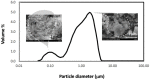
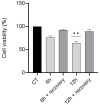
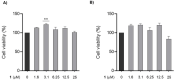
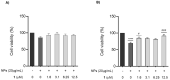
References
-
- Fontecha-Umaña F., Ríos-Castillo A.G., Ripolles-Avila C., Rodríguez-Jerez J.J. Antimicrobial Activity and Prevention of Bacterial Biofilm Formation of Silver and Zinc Oxide Nanoparticle-Containing Polyester Surfaces at Various Concentrations for Use. Foods. 2020;9:442. doi: 10.3390/foods9040442. - DOI - PMC - PubMed
Grants and funding
- CENTRO-01-0246-FEDER-000018/MEDISIS PROJECT
- UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020/Project CICECO-Aveiro Institute of Materials
- UIDB/50006/2020/LAQV-REQUIMTE-financed by national funds through the FCT/MEC (PIDDAC)
- UIDB/04501/2020 and UIDP/04501/2020/Institute of Biomedicine (iBiMED)--financed by national funds through the FCT/MEC (PIDDAC)
- CEECINST/00026/2018/FCT under the Scientific Employment Stimulus - Institutional Call (Vera L.M.Silva)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

