Phytate Hydrolysate Differently Modulates the Immune Response of Human Healthy and Cancer Colonocytes to Intestinal Bacteria
- PMID: 36296918
- PMCID: PMC9610475
- DOI: 10.3390/nu14204234
Phytate Hydrolysate Differently Modulates the Immune Response of Human Healthy and Cancer Colonocytes to Intestinal Bacteria
Abstract
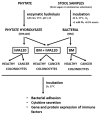
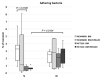
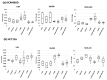
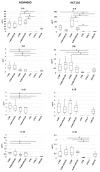
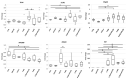
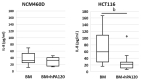
(1) Phytic acid (PA) is a component of cereal seeds and legumes, therefore its consumption is much higher in a vegan and vegetarian diet compared to a conventional diet. The diet is the main driver of metabolic activity of gut microbiota, therefore, the ability to degrade phytates by the microbiota of vegans significantly exceeds that of the gut microbiota of omnivores. The aim of the study was to investigate the early phase of the immune response of colonocytes treated with an enzymatic hydrolysate of phytic acid (hPA120) and gut bacteria. (2) Cell lines derived from healthy (NCM460D) and cancer (HCT116) colonic tissue and fecal bacteria from vegan (V) and omnivorous (O) donors were investigated. Fecal bacteria were grown in mucin and phytic acid supplemented medium. Cultured bacteria (BM) were loaded onto colonocytes alone (V BM and O BM) or in combination with the phytate hydrolysate (V BM + hPA120 and O BM + hPA120). After a treatment of 2 h, bacterial adhesion, secretion of cytokines, and the expression of genes and proteins important for immune response were determined. (3) All bacteria-treated colonocytes increased the expression of IL8 compared to controls. The significant increase of the secreted IL-8 (p < 0.01) in both cell lines was observed for O BM and O BM + hPA120. The increase of TNF, IL-1β, and IL-10 secretion in healthy colonocytes (V BM alone and with hPA120 treatments; p < 0.05) and for TNF and IL-10 in cancer cells (treatments except O BM + hPA120 and V BM, respectively; p > 0.05) were stated. A comparison of solely the effect of hPA120 on bacteria-treated colonocytes (BM vs. BM + hPA120) showed that hPA120 decreased expression of NFkB1 and TNFR (p < 0.001) in healthy colonocytes. In cancer colonocytes, the expression of TLR4 and IL1R increased after BM + hPA120 treatment, whereas the secretion of IL-8 and MYD88 and TNFR expression decreased (p < 0.01). (4) The investigated hPA120 showed a differentiated modulatory activity on the immune response of healthy and cancer human colonocytes. Especially when analyzed independently on the gut bacteria origin, it reduced the proinflammatory response of HCT116 cells to gut bacteria, while being neutral for the bacteria-treated healthy colonocytes.
Keywords: colonocytes; conventional diet; gut microbiota; immune response; inositol phosphates; phytate hydrolysate; vegan diet.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures


Similar articles
-
Phytate and Butyrate Differently Influence the Proliferation, Apoptosis and Survival Pathways in Human Cancer and Healthy Colonocytes.Nutrients. 2021 May 31;13(6):1887. doi: 10.3390/nu13061887. Nutrients. 2021. PMID: 34072741 Free PMC article.
-
Phytic acid modulates in vitro IL-8 and IL-6 release from colonic epithelial cells stimulated with LPS and IL-1beta.Dig Dis Sci. 2007 Jan;52(1):93-102. doi: 10.1007/s10620-006-9320-0. Epub 2006 Dec 12. Dig Dis Sci. 2007. PMID: 17160716
-
Diet shapes the ability of human intestinal microbiota to degrade phytate--in vitro studies.J Appl Microbiol. 2013 Jul;115(1):247-59. doi: 10.1111/jam.12204. Epub 2013 Apr 16. J Appl Microbiol. 2013. PMID: 23551617
-
Is a vegan or a vegetarian diet associated with the microbiota composition in the gut? Results of a new cross-sectional study and systematic review.Crit Rev Food Sci Nutr. 2020;60(17):2990-3004. doi: 10.1080/10408398.2019.1676697. Epub 2019 Oct 21. Crit Rev Food Sci Nutr. 2020. PMID: 31631671
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
Cited by
-
Complexification of In Vitro Models of Intestinal Barriers, A True Challenge for a More Accurate Alternative Approach.Int J Mol Sci. 2023 Feb 10;24(4):3595. doi: 10.3390/ijms24043595. Int J Mol Sci. 2023. PMID: 36835003 Free PMC article. Review.
-
The Risk of Breast Cancer between Western and Mediterranean Dietary Patterns.Nutrients. 2023 Apr 25;15(9):2057. doi: 10.3390/nu15092057. Nutrients. 2023. PMID: 37432206 Free PMC article. Review.
-
The role of dietary prehabilitation on anastomotic healing.Curr Opin Clin Nutr Metab Care. 2023 Sep 1;26(5):470-475. doi: 10.1097/MCO.0000000000000956. Epub 2023 Jun 20. Curr Opin Clin Nutr Metab Care. 2023. PMID: 37389468 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

