Effects of Whey Peptide Supplementation on Sarcopenic Obesity in High-Fat Diet-Fed Mice
- PMID: 36297085
- PMCID: PMC9611493
- DOI: 10.3390/nu14204402
Effects of Whey Peptide Supplementation on Sarcopenic Obesity in High-Fat Diet-Fed Mice
Abstract
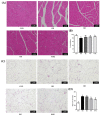
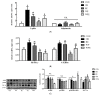
The incidence of sarcopenic obesity gradually increased in parallel with the aged population. This research examined the effects of whey peptide (WP) supplementation with/without resistant exercise (RE) on sarcopenic obesity. Male 8-month-old C57BL/6J mice were fed a control diet (10 kcal% fat) or a high-fat diet (60 kcal% fat) for 8 weeks. High-fat diet-fed mice were randomly divided into four groups: obesity control group (OB), RE (RE only), WP (WP only), and WPE (RE and WP). WP supplementation (1500 mg/day/kg B.W.) gavage and RE (ladder climbing, five times weekly, 8−10 repetitions, 10−20% B.W. load) were conducted for an additional 8 weeks. Protein and mRNA levels of markers related to energy, protein, and lipid metabolism were analyzed in skeletal muscle and adipose tissue by one-way analysis of variance (ANOVA). WP supplementation regardless of RE significantly suppressed the increasing fat mass (p = 0.016) and decreasing lean mass (p = 0.014) and alleviated abnormal morphological changes in skeletal muscle and adipose tissue (p < 0.001). In adipose tissue, WP supplementation regardless of RE ameliorated dysregulated energy metabolism and contributed to the reduction in adipocyte differentiation (PPAR-γ (p = 0.017), C/EBPα (p = 0.034)). In skeletal muscle, WP supplementation regardless of RE alleviated energy metabolism dysregulation and resulted in down-regulated protein degradation (Atrogin-1 (p = 0.003), MuRF1 (p = 0.006)) and apoptosis (Bax) (p = 0.004). Taken together, the current study elucidated that WP supplementation regardless of RE has potential anti-obesity and anti-sarcopenic effects in sarcopenic obesity.
Keywords: adipocyte differentiation; adipogenesis; protein degradation; resistant exercise; sarcopenic obesity; whey peptide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Zhang X., Xie X., Dou Q., Liu C., Zhang W., Yang Y., Deng R., Cheng A.S.K. Association of Sarcopenic Obesity with the Risk of All-Cause Mortality among Adults over a Broad Range of Different Settings: A Updated Meta-Analysis. BMC Geriatr. 2019;19:183. doi: 10.1186/s12877-019-1195-y. - DOI - PMC - PubMed
-
- CDC . Obesity is a Common, Serious, and Costly Disease. CDC; Atlanta, GA, USA: 2020.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

