Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS
- PMID: 36297099
- PMCID: PMC9610384
- DOI: 10.3390/nu14204415
Biological Effects of Intravenous Vitamin C on Neutrophil Extracellular Traps and the Endothelial Glycocalyx in Patients with Sepsis-Induced ARDS
Abstract
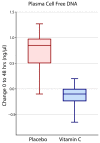
(1) Background: The disease-modifying mechanisms of high-dose intravenous vitamin C (HDIVC) in sepsis induced acute respiratory distress syndrome (ARDS) is unclear. (2) Methods: We performed a post hoc study of plasma biomarkers from subjects enrolled in the randomized placebo-controlled trial CITRIS-ALI. We explored the effects of HDIVC on cell-free DNA (cfDNA) and syndecan-1, surrogates for neutrophil extracellular trap (NET) formation and degradation of the endothelial glycocalyx, respectively. (3) Results: In 167 study subjects, baseline cfDNA levels in HDIVC (84 subjects) and placebo (83 subjects) were 2.18 ng/µL (SD 4.20 ng/µL) and 2.65 ng/µL (SD 3.87 ng/µL), respectively, p = 0.45. At 48-h, the cfDNA reduction was 1.02 ng/µL greater in HDIVC than placebo, p = 0.05. Mean baseline syndecan-1 levels in HDIVC and placebo were 9.49 ng/mL (SD 5.57 ng/mL) and 10.83 ng/mL (SD 5.95 ng/mL), respectively, p = 0.14. At 48 h, placebo subjects exhibited a 1.53 ng/mL (95% CI, 0.96 to 2.11) increase in syndecan-1 vs. 0.75 ng/mL (95% CI, 0.21 to 1.29, p = 0.05), in HDIVC subjects. (4) Conclusions: HDIVC infusion attenuated cell-free DNA and syndecan-1, biomarkers associated with sepsis-induced ARDS. Improvement of these biomarkers suggests amelioration of NETosis and shedding of the vascular endothelial glycocalyx, respectively.
Keywords: acute respiratory distress syndrome; cell-free DNA; glycocalyx; sepsis; syndecan-1; vitamin C.
Conflict of interest statement
M.G.K. received funding from Xellia Pharmaceuticals for serving in the advisory board. J.D.T. received industry-academic funding from Olympus Spiration for serving on a DSMB. G.S.M. received industry-academic funding from The Marcus Foundation, Bristol-Myers Squibb, Cheetah Medical, and Grifols research advisory board funds. J.E.S. received industry-academic funding from The Marcus Foundation, and BARDA. All other authors declare no competing interest.
Figures

References
-
- Bellani G., Laffey J.G., Pham T., Fan E., Brochard L., Esteban A., Gattinoni L., Van Haren F., Larsson A., McAuley D.F., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. - DOI - PubMed
-
- Fowler A.A., III, Syed A.A., Knowlson S., Sculthorpe R., Farthing D., Dewilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

