Inhibition Effects of Nippostrongylus brasiliensis and Its Derivatives against Atherosclerosis in ApoE-/- Mice through Anti-Inflammatory Response
- PMID: 36297265
- PMCID: PMC9610917
- DOI: 10.3390/pathogens11101208
Inhibition Effects of Nippostrongylus brasiliensis and Its Derivatives against Atherosclerosis in ApoE-/- Mice through Anti-Inflammatory Response
Abstract
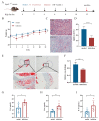
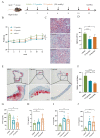
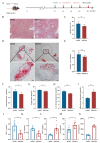
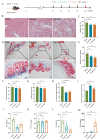
Atherosclerosis (AS) is a dominant and growing cause of death and disability worldwide that involves inflammation from its inception to the emergence of complications. Studies have demonstrated that intervention with helminth infections or derived products could modulate the host immune response and effectively prevent or mitigate the onset and progression of inflammation-related diseases. Therefore, to understand the molecular mechanisms underlying the development of atherosclerosis, we intervened in ApoE-/- mice maintained on a high-fat diet with Nippostrongylus brasiliensis (N. brasiliensis) infection and immunized with its derived products. We found that N. brasiliensis infection and its derived proteins had suitable protective effects both in the initial and progressive stages of atherosclerosis, effectively reducing aortic arch plaque areas and liver lipid contents and downregulating serum LDL levels, which may be associated with the significant upregulation of serum anti-inflammatory cytokines (IL-10 and IL-4) and the down-regulation of proinflammatory cytokines (TNF-α and IFN-γ) in the serum. In conclusion, these data highlighted the effective regulatory role of N. brasiliensis and its derived proteins in the development and progression of atherosclerosis. This could provide a promising new avenue for the prevention and treatment of atherosclerosis.
Keywords: Nippostrongylus brasiliensis; apolipoprotein-E-deficient mouse; atherosclerosis; hookworm; intervention; protective effect.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model.Biomolecules. 2024 Jun 8;14(6):672. doi: 10.3390/biom14060672. Biomolecules. 2024. PMID: 38927075 Free PMC article.
-
[Polarization of human acute monocytic leukemia THP-1 cells-derived macprophages induced by Nippostrongylus brasiliensis proteins in vitro].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020 Jul 10;32(4):367-373. doi: 10.16250/j.32.1374.2020003. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020. PMID: 32935510 Chinese.
-
Mining Anti-Inflammation Molecules From Nippostrongylus brasiliensis-Derived Products Through the Metabolomics Approach.Front Cell Infect Microbiol. 2021 Nov 11;11:781132. doi: 10.3389/fcimb.2021.781132. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34858883 Free PMC article.
-
MCS-18, a natural product isolated from Helleborus purpurascens, inhibits maturation of dendritic cells in ApoE-deficient mice and prevents early atherosclerosis progression.Atherosclerosis. 2014 Aug;235(2):263-72. doi: 10.1016/j.atherosclerosis.2014.05.915. Epub 2014 May 14. Atherosclerosis. 2014. PMID: 24887015
-
The role of ILC2 in hookworm infection.Parasite Immunol. 2018 Feb;40(2). doi: 10.1111/pim.12429. Epub 2017 May 22. Parasite Immunol. 2018. PMID: 28369954 Review.
Cited by
-
Helminth-derived biomolecules as potential therapeutics against ulcerative colitis.Immunotherapy. 2024;16(10):635-640. doi: 10.1080/1750743X.2024.2360382. Epub 2024 Jun 18. Immunotherapy. 2024. PMID: 38888436 Free PMC article. No abstract available.
-
Helminth-derived molecules: Pathogenic and pharmacopeial roles.J Biomed Res. 2024 Sep 24;38(6):547-568. doi: 10.7555/JBR.38.20240177. J Biomed Res. 2024. PMID: 39314046 Free PMC article.
-
Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model.Biomolecules. 2024 Jun 8;14(6):672. doi: 10.3390/biom14060672. Biomolecules. 2024. PMID: 38927075 Free PMC article.
-
Helminth-induced immune modulation in colorectal cancer: exploring therapeutic applications.Front Immunol. 2025 Apr 14;16:1484686. doi: 10.3389/fimmu.2025.1484686. eCollection 2025. Front Immunol. 2025. PMID: 40297577 Free PMC article. Review.
References
-
- Poels K., van Leent M.M.T., Boutros C., Tissot H., Roy S., Meerwaldt A.E., Toner Y.C.A., Reiche M.E., Kusters P.J.H., Malinova T., et al. Immune Checkpoint Inhibitor Therapy Aggravates T Cell-Driven Plaque Inflammation in Atherosclerosis. JACC CardioOncol. 2020;2:599–610. doi: 10.1016/j.jaccao.2020.08.007. - DOI - PMC - PubMed
-
- Chan Y.H., Ramji D.P. Key Roles of Inflammation in Atherosclerosis: Mediators Involved in Orchestrating the Inflammatory Response and Its Resolution in the Disease Along with Therapeutic Avenues Targeting Inflammation. Methods Mol. Biol. 2022;2419:21–37. doi: 10.1007/978-1-0716-1924-7_2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

