Adsorption of Chlormethine Anti-Cancer Drug on Pure and Aluminum-Doped Boron Nitride Nanocarriers: A Comparative DFT Study
- PMID: 36297293
- PMCID: PMC9607567
- DOI: 10.3390/ph15101181
Adsorption of Chlormethine Anti-Cancer Drug on Pure and Aluminum-Doped Boron Nitride Nanocarriers: A Comparative DFT Study
Abstract
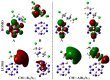
The efficacy of pure and aluminum (Al)-doped boron nitride nanocarriers (B12N12 and AlB11N12) in adsorbing Chlormethine (CM), an anti-cancer drug, was comparatively dissected by means of the density functional theory method. The CM∙∙∙B12N12 and ∙∙∙AlB11N12 complexes were studied within two configurations, A and B, in which the adsorption process occurred via N∙∙∙ and Cl∙∙∙B/Al interactions, respectively. The electrostatic potential affirmations confirmed the opulent ability of the studied nanocarriers to engage in delivering CM via two prominent electrophilic sites (B and Al). Furthermore, the adsorption process within the CM∙∙∙AlB11N12 complexes was noticed to be more favorable compared to that within the CM∙∙∙B12N12 analog and showed interaction and adsorption energy values up to -59.68 and -52.40 kcal/mol, respectively, for configuration A. Symmetry-adapted perturbation theory results indicated that electrostatic forces were dominant in the adsorption process. Notably, the adsorption of CM over B12N12 and AlB11N12 nanocarriers exhibited predominant changes in their electronic properties. An elemental alteration was also revealed for the softness and hardness of B12N12 and AlB11N12 nanocarriers before and following the CM adsorption. Spontaneity and exothermic nature were obviously observed for the studied complexes and confirmed by the negative values of thermodynamic parameters. In line with energetic manifestation, Gibbs free energy and enthalpy change were drastically increased by the Al doping process, with values raised to -37.15 and -50.14 kcal/mol, respectively, for configuration A of the CM∙∙∙AlB11N12 complex. Conspicuous enhancement was noticed for the adsorption process in the water phase more than that in the gas phase and confirmed by the negative values of the solvation energy up to -53.50 kcal/mol for configuration A of the CM∙∙∙AlB11N12 complex. The obtained outcomes would be the linchpin for the future utilization of boron nitride as a nanocarrier.
Keywords: Chlormethine; DFT calculations; anti-cancer drug; boron nitride nanocarriers; thermodynamic parameters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Mias S., Sudor J., Camon H. PNIPAM: A thermo-activated nano-material for use in optical devices. Microsyst. Technol. 2007;14:691–695. doi: 10.1007/s00542-007-0454-6. - DOI
-
- Planeix J.M., Coustel N., Coq B., Brotons V., Kumbhar P.S., Dutartre R., Geneste P., Bernier P., Ajayan P.M. Application of carbon nanotubes as supports in heterogeneous catalysis. J. Am. Chem. Soc. 1994;116:7935–7936. doi: 10.1021/ja00096a076. - DOI
-
- Varghese S.S., Lonkar S., Singh K.K., Swaminathan S., Abdala A. Recent advances in graphene based gas sensors. Sens. Actuators B Chem. 2015;218:160–183. doi: 10.1016/j.snb.2015.04.062. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

