Caulis Polygoni Multiflori Accelerates Megakaryopoiesis and Thrombopoiesis via Activating PI3K/Akt and MEK/ERK Signaling Pathways
- PMID: 36297316
- PMCID: PMC9607024
- DOI: 10.3390/ph15101204
Caulis Polygoni Multiflori Accelerates Megakaryopoiesis and Thrombopoiesis via Activating PI3K/Akt and MEK/ERK Signaling Pathways
Abstract
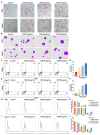
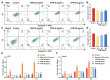
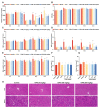
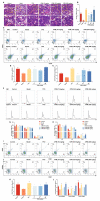
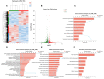
Thrombocytopenia is one of the most common complications of cancer therapy. Until now, there are still no satisfactory medications to treat chemotherapy and radiation-induced thrombocytopenia (CIT and RIT, respectively). Caulis Polygoni Multiflori (CPM), one of the most commonly used Chinese herbs, has been well documented to nourish blood for tranquilizing the mind and treating anemia, suggesting its beneficial effect on hematopoiesis. However, it is unknown whether CPM can accelerate megakaryopoiesis and thrombopoiesis. Here, we employ a UHPLC Q-Exactive HF-X mass spectrometer (UHPLC QE HF-X MS) to identify 11 ingredients in CPM. Then, in vitro experiments showed that CPM significantly increased megakaryocyte (MK) differentiation and maturation but did not affect apoptosis and lactate dehydrogenase (LDH) release of K562 and Meg-01 cells. More importantly, animal experiments verified that CPM treatment markedly accelerated platelet recovery, megakaryopoiesis and thrombopoiesis in RIT mice without hepatic and renal toxicities in vivo. Finally, RNA-sequencing (RNA-seq) and western blot were used to determine that CPM increased the expression of proteins related to PI3K/Akt and MEK/ERK (MAPK) signaling pathways. On the contrary, blocking PI3K/Akt and MEK/ERK signaling pathways with their specific inhibitors suppressed MK differentiation induced by CPM. In conclusion, for the first time, our study demonstrates that CPM may be a promised thrombopoietic agent and provide an experimental basis for expanding clinical use.
Keywords: Caulis Polygoni Multiflori; MEK/ERK; PI3K/Akt; megakaryopoiesis; thrombocytopenia; thrombopoiesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

