A Review of the Biological Activity of Amidrazone Derivatives
- PMID: 36297331
- PMCID: PMC9606871
- DOI: 10.3390/ph15101219
A Review of the Biological Activity of Amidrazone Derivatives
Abstract
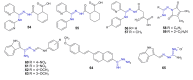
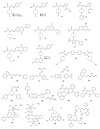
Amidrazones are widely used in chemical synthesis, industry and agriculture. We compiled some of the most important findings on the biological activities of amidrazones described in the years 2010-2022. The data were obtained using the ScienceDirect, Reaxys and Google Scholar search engines with keywords (amidrazone, carbohydrazonamide, carboximidohydrazide, aminoguanidine) and structure strategies. Compounds with significant biological activities were included in the review. The described structures derived from amidrazones include: amidrazone derivatives; aminoguanidine derivatives; complexes obtained using amidrazones as ligands; and some cyclic compounds obtained from amidrazones and/or containing an amidrazone moiety in their structures. This review includes chapters based on compound activities, including: tuberculostatic, antibacterial, antifungal, antiparasitic, antiviral, anti-inflammatory, cytoprotective, and antitumor compounds, as well as furin and acetylocholinesterase inhibitors. Detailed information on the compounds tested in vivo, along the mechanisms of action and toxicity of the selected amidrazone derivatives, are described. We describe examples of compounds that have a chance of becoming drugs due to promising preclinical or clinical research, as well as old drugs with new therapeutic targets (repositioning) which have the potential to be used in the treatment of other diseases. The described examples prove that amidrazone derivatives are a potential source of new therapeutic substances and deserve further research.
Keywords: amidrazone; aminoguanidine; anti-inflammatory; antibacterial; antifungal; antiparasitic; antitumor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

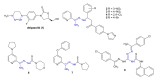
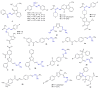
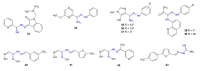
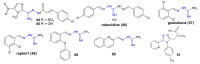




References
-
- Aly A., El-Din A.M.N. Functionality of amidines and amidrazones. Arkivoc. 2008;1:153–194. doi: 10.3998/ark.5550190.0009.106. - DOI
-
- Ben Salem A., Ben Salah B., Mhalla D., Trigui M., Mourer M., Regnouf-de-Vains J.-B., Kossentini M. Synthesis, crystal structure and biological studies of novel amidrazones, triazoles, Thiatriazole and Triazine compounds. J. Mol. Struct. 2020;1214:128209. doi: 10.1016/j.molstruc.2020.128209. - DOI
-
- Stepanov A.I., Sannikov V.S., Dashko D.V., Roslyakov A.G., Astrat’Ev A.A., Stepanova E.V. A new preparative method and some chemical properties of 4-R-furazan-3-carboxylic acid amidrazones. Chem. Heterocycl. Compd. 2015;51:350–360. doi: 10.1007/s10593-015-1707-4. - DOI
-
- Nomenclature of Organic Chemistry IUPAC Recommendations and Preferred Names 2013. [(accessed on 18 February 2022)]. Available online: https://iupac.qmul.ac.uk/BlueBook/PDF/2013BlueBook.pdf.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

