Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry
- PMID: 36297362
- PMCID: PMC9607481
- DOI: 10.3390/ph15101250
Chalcone: A Promising Bioactive Scaffold in Medicinal Chemistry
Abstract
Chalcones are a class of privileged scaffolds with high medicinal significance due to the presence of an α,β-unsaturated ketone functionality. Numerous functional modifications of chalcones have been reported, along with their pharmacological behavior. The present review aims to summarize the structures from natural sources, synthesis methods, biological characteristics against infectious and non-infectious diseases, and uses of chalcones over the past decade, and their structure-activity relationship studies are detailed in depth. This critical review provides guidelines for the future design and synthesis of various chalcones. In addition, this could be highly supportive for medicinal chemists to develop more promising candidates for various infectious and non-infectious diseases.
Keywords: Claisen–Schmidt condensation; chalcones; infectious diseases; natural sources; non-infectious diseases; structure–activity relationship.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



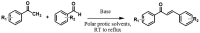




















References
-
- Narender T., Papi Reddy K. A Simple and Highly Efficient Method for the Synthesis of Chalcones by Using Borontrifluoride-Etherate. Tetrahedron Lett. 2007;48:3177–3180. doi: 10.1016/j.tetlet.2007.03.054. - DOI
-
- Mustafa M., Mostafa Y.A. A Facile Synthesis, Drug-Likeness, and in Silico Molecular Docking of Certain New Azidosulfonamide–Chalcones and Their in Vitro Antimicrobial Activity. Mon. Für Chem. Chem. Mon. 2020;151:417–427. doi: 10.1007/s00706-020-02568-8. - DOI
Publication types
Grants and funding
- NRF-2022R1I1A1A01068652/National Research Foundation of Korea
- NRF-2022R1I1A3069687/National Research Foundation of Korea
- NRF-2022R1I1A1A01069734/National Research Foundation of Korea
- NRF-2022R1A2C2005057/National Research Foundation of Korea
- NRF-2022R1A2C2005057/National Research Foundation of Korea
LinkOut - more resources
Full Text Sources
Miscellaneous

