Applications of Exosomes in Diagnosing Muscle Invasive Bladder Cancer
- PMID: 36297462
- PMCID: PMC9607910
- DOI: 10.3390/pharmaceutics14102027
Applications of Exosomes in Diagnosing Muscle Invasive Bladder Cancer
Abstract
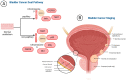
Muscle Invasive Bladder Cancer (MIBC) is a subset of bladder cancer with a significant risk for metastases and death. It accounts for nearly 25% of bladder cancer diagnoses. A diagnostic work-up for MIBC is inclusive of urologic evaluation, radiographic imaging with a CT scan, urinalysis, and cystoscopy. These evaluations, especially cystoscopy, are invasive and carry the risk of secondary health concerns. Non-invasive diagnostics such as urine cytology are an attractive alternative currently being investigated to mitigate the requirement for cystoscopy. A pitfall in urine cytology is the lack of available options with high reliability, specificity, and sensitivity to malignant bladder cells. Exosomes are a novel biomarker source which could resolve some of the concerns with urine cytology, due to the high specificity as the surrogates of tumor cells. This review serves to define muscle invasive bladder cancer, current urine cytology methods, the role of exosomes in MIBC, and exosomes application as a diagnostic tool in MIBC. Urinary exosomes as the specific populations of extracellular vesicles could provide additional biomarkers with specificity and sensitivity to bladder malignancies, which are a consistent source of cellular information to direct clinicians for developing treatment strategies. Given its strong presence and differentiation ability between normal and cancerous cells, exosome-based urine cytology is highly promising in providing a perspective of a patient's bladder cancer.
Keywords: biomarkers; bladder cancer diagnosis; bladder cancer screening; exosomes; muscle invasive bladder cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic.BJU Int. 2012 Jul;110(1):84-94. doi: 10.1111/j.1464-410X.2011.10664.x. Epub 2011 Nov 28. BJU Int. 2012. PMID: 22122739
-
Urine exosomes as biomarkers in bladder cancer diagnosis and prognosis: From functional roles to clinical significance.Front Oncol. 2022 Sep 20;12:1019391. doi: 10.3389/fonc.2022.1019391. eCollection 2022. Front Oncol. 2022. PMID: 36203422 Free PMC article. Review.
-
The role of fluorescence in situ hybridization assay for surveillance of non-muscle invasive bladder cancer.Can J Urol. 2010 Apr;17(2):5077-81. Can J Urol. 2010. PMID: 20398445
-
Follow-up in non-muscle-invasive bladder cancer-International Bladder Cancer Network recommendations.Urol Oncol. 2016 Oct;34(10):460-8. doi: 10.1016/j.urolonc.2016.05.028. Epub 2016 Jun 29. Urol Oncol. 2016. PMID: 27368880 Review.
-
The role of urine markers, white light cystoscopy and fluorescence cystoscopy in recurrence, progression and follow-up of non-muscle invasive bladder cancer.World J Urol. 2014 Jun;32(3):651-9. doi: 10.1007/s00345-013-1035-1. Epub 2013 Oct 29. World J Urol. 2014. PMID: 24166285 Review.
Cited by
-
Spontaneous Necrosis of a High-Risk Bladder Tumor Under Immunotherapy for Concurrent Malignant Melanoma: Role of BRAF Mutations and PD-L1 Expression.Biomedicines. 2025 Feb 5;13(2):377. doi: 10.3390/biomedicines13020377. Biomedicines. 2025. PMID: 40002790 Free PMC article.
-
The Current Progress and Future Options of Multiple Therapy and Potential Biomarkers for Muscle-Invasive Bladder Cancer.Biomedicines. 2023 Feb 13;11(2):539. doi: 10.3390/biomedicines11020539. Biomedicines. 2023. PMID: 36831075 Free PMC article. Review.
-
Malignant mesothelioma tumours: molecular pathogenesis, diagnosis, and therapies accompanying clinical studies.Front Oncol. 2023 Jul 4;13:1204722. doi: 10.3389/fonc.2023.1204722. eCollection 2023. Front Oncol. 2023. PMID: 37469419 Free PMC article. Review.
-
Urinary exosomal lnc-TAF12-2:1 promotes bladder cancer progression through the miR-7847-3p/ASB12 regulatory axis.Genes Dis. 2024 Aug 5;12(4):101384. doi: 10.1016/j.gendis.2024.101384. eCollection 2025 Jul. Genes Dis. 2024. PMID: 40297540 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

