Disialoganglioside GD2-Targeted Near-Infrared Photoimmunotherapy (NIR-PIT) in Tumors of Neuroectodermal Origin
- PMID: 36297471
- PMCID: PMC9612122
- DOI: 10.3390/pharmaceutics14102037
Disialoganglioside GD2-Targeted Near-Infrared Photoimmunotherapy (NIR-PIT) in Tumors of Neuroectodermal Origin
Abstract
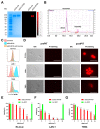
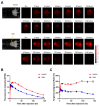
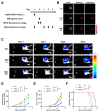
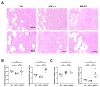
Disialoganglioside (GD2) is a subtype of glycolipids that is highly expressed in tumors of neuroectodermal origins, such as neuroblastoma and osteosarcoma. Its limited expression in normal tissues makes GD2 a potential target for precision therapy. Several anti-GD2 monoclonal antibodies are currently in clinical use and have had moderate success. Near-infrared photoimmunotherapy (NIR-PIT) is a cancer therapy that arms antibodies with IRDye700DX (IR700) and then exposes this antibody-dye conjugate (ADC) to NIR light at a wavelength of 690 nm. NIR light irradiation induces a profound photochemical response in IR700, resulting in protein aggregates that lead to cell membrane damage and death. In this study, we examined the feasibility of GD2-targeted NIR-PIT. Although GD2, like other glycolipids, is only located in the outer leaflet of the cell membrane, the aggregates formation exerted sufficient physical force to disrupt the cell membrane and kill target cells in vitro. In in vivo studies, tumor growth was significantly inhibited after GD2-targeted NIR-PIT, resulting in prolonged survival. Following GD2-targeted NIR-PIT, activation of host immunity was observed. In conclusion, GD2-targeted NIR-PIT was similarly effective to the conventional protein-targeted NIR-PIT. This study demonstrates that membrane glycolipid can be a new target of NIR-PIT.
Keywords: IR700; ganglioside; glycosphingolipid; immunotherapy; neuroblastoma.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Near-Infrared Photoimmunotherapy of Cancer.Acc Chem Res. 2019 Aug 20;52(8):2332-2339. doi: 10.1021/acs.accounts.9b00273. Epub 2019 Jul 23. Acc Chem Res. 2019. PMID: 31335117 Free PMC article. Review.
-
Near-Infrared Photochemoimmunotherapy by Photoactivatable Bifunctional Antibody-Drug Conjugates Targeting Human Epidermal Growth Factor Receptor 2 Positive Cancer.Bioconjug Chem. 2017 May 17;28(5):1458-1469. doi: 10.1021/acs.bioconjchem.7b00144. Epub 2017 Apr 26. Bioconjug Chem. 2017. PMID: 28402624 Free PMC article.
-
Near-infrared photoimmunotherapy with galactosyl serum albumin in a model of diffuse peritoneal disseminated ovarian cancer.Oncotarget. 2016 Nov 29;7(48):79408-79416. doi: 10.18632/oncotarget.12710. Oncotarget. 2016. PMID: 27765903 Free PMC article.
-
Fluorescence Imaging of Tumor-Accumulating Antibody-IR700 Conjugates Prior to Near-Infrared Photoimmunotherapy (NIR-PIT) Using a Commercially Available Camera Designed for Indocyanine Green.Mol Pharm. 2021 Mar 1;18(3):1238-1246. doi: 10.1021/acs.molpharmaceut.0c01107. Epub 2021 Jan 27. Mol Pharm. 2021. PMID: 33502869 Free PMC article.
-
Near-Infrared Photoimmunotherapy in Brain Tumors-An Unexplored Frontier.Pharmaceuticals (Basel). 2025 May 19;18(5):751. doi: 10.3390/ph18050751. Pharmaceuticals (Basel). 2025. PMID: 40430568 Free PMC article. Review.
Cited by
-
Chimeric Antigen Receptor T Cell and Chimeric Antigen Receptor NK Cell Therapy in Pediatric and Adult High-Grade Glioma-Recent Advances.Cancers (Basel). 2024 Jan 31;16(3):623. doi: 10.3390/cancers16030623. Cancers (Basel). 2024. PMID: 38339374 Free PMC article. Review.
-
Targeting Pathways in Neuroblastoma: Advances in Treatment Strategies and Clinical Outcomes.Int J Mol Sci. 2025 May 15;26(10):4722. doi: 10.3390/ijms26104722. Int J Mol Sci. 2025. PMID: 40429864 Free PMC article. Review.
-
Region-specific mouse brain ganglioside distribution revealed by an improved isobaric aminoxyTMT labeling strategy with automated data processing.Anal Bioanal Chem. 2023 Dec;415(29-30):7269-7279. doi: 10.1007/s00216-023-04995-y. Epub 2023 Oct 20. Anal Bioanal Chem. 2023. PMID: 37857739 Free PMC article.
-
Near-infrared photoimmunotherapy targeting PD-L1: Improved efficacy by preconditioning the tumor microenvironment.Cancer Sci. 2024 Jul;115(7):2396-2409. doi: 10.1111/cas.16195. Epub 2024 Apr 26. Cancer Sci. 2024. PMID: 38671582 Free PMC article.
-
Glycosylation in cancer: mechanisms, diagnostic markers, and therapeutic applications.Mol Cell Biochem. 2025 May 19. doi: 10.1007/s11010-025-05303-1. Online ahead of print. Mol Cell Biochem. 2025. PMID: 40389792 Review.
References
-
- Mujoo K., A Cheresh D., Yang H.M., A Reisfeld R. Disialoganglioside GD2 on human neuroblastoma cells: Target antigen for monoclonal antibody-mediated cytolysis and suppression of tumor growth. Cancer Res. 1987;47:1098–1104. - PubMed
-
- Heiner J.P., Miraldi F., Kallick S., Makley J., Neely J., Smith-Mensah W.H., Cheung N.K. Localization of GD2-specific monoclonal antibody 3F8 in human osteosarcoma. Cancer Res. 1987;47:5377–5381. - PubMed
-
- Lipinski M., Braham K., Philip I., Wiels J., Philip T., Goridis C., Lenoir G.M., Tursz T. Neuroectoderm-associated antigens on Ewing’s sarcoma cell lines. Cancer Res. 1987;47:183–187. - PubMed
-
- Cheresh A.D., Rosenberg J., Mujoo K., Hirschowitz L., Reisfeld A.R. Biosynthesis and expression of the disialoganglioside GD2, a relevant target antigen on small cell lung carcinoma for monoclonal antibody-mediated cytolysis. Cancer Res. 1986;46:5112–5118. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

