Penicillin and Oxacillin Loaded on PEGylated-Graphene Oxide to Enhance the Activity of the Antibiotics against Methicillin-Resistant Staphylococcus aureus
- PMID: 36297484
- PMCID: PMC9607092
- DOI: 10.3390/pharmaceutics14102049
Penicillin and Oxacillin Loaded on PEGylated-Graphene Oxide to Enhance the Activity of the Antibiotics against Methicillin-Resistant Staphylococcus aureus
Abstract
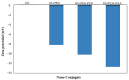
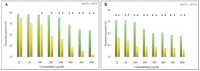
Infectious diseases are known as the second biggest cause of death worldwide, due to the development of antibiotic resistance. To overcome this problem, nanotechnology offers some promising approaches, such as drug delivery systems that can enhance drug efficiency. Herein, a Graphene Oxide-polyethylene glycol (GO-PEG) nano-platform was synthesized and penicillin and oxacillin, two antibiotics that are ineffective against Methicillin-resistant S. aureus (MRSA), were loaded on it to improve their effectiveness. The nanocomposites were characterized using FTIR, XRD, UV-Vis, FE-SEM/EDX, and Zeta potential analyses, followed by an evaluation of their antibacterial activity toward MRSA. Based on the results, drug loaded GO-PEG nanocomposites with loading efficiencies of 81% and 92% for penicillin and oxacillin, respectively, were successfully synthesized. They showed a controlled release within six days. The zeta potential of GO-PEG-oxacillin and penicillin was -13 mV and -11 mV, respectively. The composites showed much more activity against MRSA (80-85% inhibition) in comparison to GO-PEG (almost 0% inhibition) and pure antibiotics (40-45% inhibition). SEM images of MRSA treated with GO-PEG-antibiotics showed a deformation in the structure of bacterial cells, which led to the collapse of their intracellular components. These results demonstrate the effectiveness of utilizing the GO-based nanoplatforms in enhancing the antibacterial activity of the antibiotics.
Keywords: antibiotic resistance; graphene oxide; methicillin-resistant Staphylococcus aureus; oxacillin; penicillin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Odonkor S.T., Addo K.K. Bacteria Resistance to Antibiotics: Recent Trends and Challenges. Int. J. Biol. Med. Res. 2011;2:1204–1210.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

