Platinum(IV) Complexes of the 1,3,5-Triamino Analogue of the Biomolecule Cis-Inositol Designed as Innovative Antineoplastic Drug Candidates
- PMID: 36297500
- PMCID: PMC9611922
- DOI: 10.3390/pharmaceutics14102057
Platinum(IV) Complexes of the 1,3,5-Triamino Analogue of the Biomolecule Cis-Inositol Designed as Innovative Antineoplastic Drug Candidates
Abstract
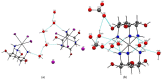
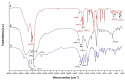
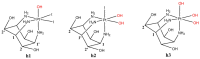
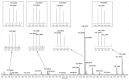
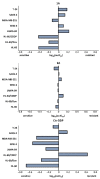
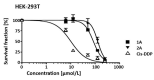
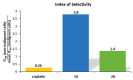
Metal complexes occupy a special place in the field of treatment and diagnostics. Their main advantages stem from the possibility of fine-tuning their thermodynamic properties and kinetic behavior in the biological milieu by applying different approaches such as properly constructed inner coordination sphere, appropriate choice of ligands, metal oxidation state, redox potential, etc., which are specific to these compounds. Here we discuss the design and synthesis of two octahedral cationic Pt(IV) complexes of the tridentate ligand all-cis-2,4,6-triaminocyclohexane-1,3,5-triol (taci) with composition, fac-[Pt(taci)I3]+, 1 and bis-[Pt(taci)2]4+, 2 as well as the potential for their application as antineoplastic agents. The complexes have been isolated in a solid state as: fac-[Pt(taci)I3]I·3H2O (1A), fac-[Pt(taci)I3]I (1B), fac-[Pt(taci)I3]I·2DMF (1C), bis-[Pt(taci)2](CO3)2·6H2O (2A) by changing the acidity of the reaction systems, the molar ratios of the reagents and the counterions, and by re-crystallization. The ligand taci is coordinated through the NH2-groups, each molecule occupying three coordination places in the inner coordination sphere of Pt(IV). Monitoring of the hydrolysis processes of 1A and 2A at different acidity showed that while 2A remained stable over the study period, the I--ions in 1A were successively substituted, with the main product under physiologically mimetic conditions being fac,cis-[Pt(taci)I(OH)2]+ (h2). The antiproliferative tests involved eight cancer cell models, among which chemosensitive (derived from leukemias and solid tumors) and chemoresistant human Acute myeloid leukemia lines (HL-60/Dox, HL-60/CDDP), as well as the non-malignant kidney' cells HEK-293T showed that the complexes 1A and 2A are characterized by a fundamentally different profile of chemosensitivity and spectrum of cytotoxic activity compared to cisplatin. The new Pt(IV) complexes were shown to be more effective in selectively inhibiting the proliferation of human malignant cells compared to cisplatin. Remarkable activity was recorded for 1A, which showed an effect (IC50 = 8.9 ± 2.4) at more than 16-fold lower concentration than cisplatin (IC50 = 144.4 ± 9.8) against the resistant cell line HL-60/CDDP. In parallel, 1A exhibited virtually the same cytotoxic effect against the parental HL-60 cells (IC50 = 9.0 ± 1.2), where cisplatin displays comparable chemosensitivity (IC50 = 8.3 ± 0.8). The determined resistance indices (RI~1) show unequivocally that the resistant lines are sensitive to both compounds tested; therefore, they are capable of overcoming the mechanisms of cisplatin resistance. The structural features of these compounds and their promising pharmacological properties justify their inclusion in the group of "non-classical metal-based antitumor compounds" and are a prerequisite for the admission of alternative mechanisms of action.
Keywords: Pt(IV) complexes; biological activity; cisplatin resistance; novel metal-based drugs; structure-activity studies; synthesis and characterization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

