Preparation of pH-Responsive Hydrogels Based on Chondroitin Sulfate/Alginate for Oral Drug Delivery
- PMID: 36297545
- PMCID: PMC9606947
- DOI: 10.3390/pharmaceutics14102110
Preparation of pH-Responsive Hydrogels Based on Chondroitin Sulfate/Alginate for Oral Drug Delivery
Abstract
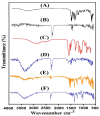
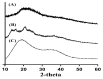
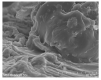
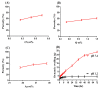
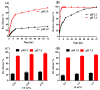
This study investigates pH-sensitive hydrogels based on biocompatible, biodegradable polysaccharides and natural polymers such as chondroitin sulfate and alginate in combination with synthetic monomer such as acrylic acid, as controlled drug carriers. Investigations were conducted for chondroitin sulfate/alginate-graft-poly(acrylic acid) hydrogel in various mixing ratios of chondroitin sulfate, alginate and acrylic acid in the presence of ammonium persulfate and N',N'-Methylene bisacrylamide. Crosslinking and loading of drug were confirmed by Fourier transform infrared spectroscopy. Thermal stability of both polymers was enhanced after crosslinking as indicated by thermogravimetric analysis and differential scanning calorimeter thermogram of developed hydrogel. Similarly, surface morphology was evaluated by scanning electron microscopy, whereas crystallinity of the polymers and developed hydrogel was investigated by powder X-ray diffraction. Furthermore, swelling and drug-release studies were investigated in acidic and basic medium of pH 1.2 and 7.4 at 37 °C, respectively. Maximum swelling and drug release were detected at pH 7.4 as compared to pH 1.2. Increased incorporation of hydrogel contents led to an increase in porosity, drug loading, and gel fraction while a reduction in sol fraction was seen. The polymer volume fraction was found to be low at pH 7.4 compared to pH 1.2, indicating a prominent and greater swelling of the prepared hydrogels at pH 7.4. Likewise, a biodegradation study revealed a slow degradation rate of the developed hydrogel. Hence, we can conclude from the results that a fabricated system of hydrogel could be used as a suitable carrier for the controlled delivery of ketorolac tromethamine.
Keywords: biodegradation study; drug release; hydrogels; porosity; swelling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Wagh P., Mujumdar A., Naik J.B. Preparation and characterization of ketorolac tromethamine-loaded ethyl cellulose micro-/nanospheres using different techniques. Part. Sci. Technol. 2019;37:347–357. doi: 10.1080/02726351.2017.1383330. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

