Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing
- PMID: 36297560
- PMCID: PMC9611271
- DOI: 10.3390/pharmaceutics14102125
Continuous NPWT Regulates Fibrosis in Murine Diabetic Wound Healing
Abstract
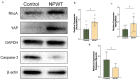
Scarring is associated with significant morbidity. The mechanical signaling factor yes-associated protein (YAP) has been linked to Engrailed-1 (En1)-lineage positive fibroblasts (EPFs), a pro-scarring fibroblast lineage, establishing a connection between mechanotransduction and fibrosis. In this study, we investigate the impact of micromechanical forces exerted through negative pressure wound therapy (NPWT) on the pathophysiology of fibrosis. Full-thickness excisional dorsal skin wounds were created on diabetic (db/db) mice which were treated with occlusive covering (control) or NPWT (continuous, −125 mmHg, 7 days; NPWT). Analysis was performed on tissue harvested 10 days after wounding. NPWT was associated with increased YAP (p = 0.04) but decreased En1 (p = 0.0001) and CD26 (p < 0.0001). The pro-fibrotic factors Vimentin (p = 0.04), α-SMA (p = 0.04) and HSP47 (p = 0.0008) were decreased with NPWT. Fibronectin was higher (p = 0.01) and collagen deposition lower in the NPWT group (p = 0.02). NPWT increased cellular proliferation (p = 0.002) and decreased apoptosis (p = 0.03). Western blotting demonstrated increased YAP (p = 0.02) and RhoA (p = 0.03) and decreased Caspase-3 (p = 0.03) with NPWT. NPWT uncouples YAP from EPF activation, through downregulation of Caspace-3, a pro-apoptotic factor linked to keloid formation. Mechanotransduction decreases multiple pro-fibrotic factors. Through this multifactorial process, NPWT significantly decreases fibrosis and offers promising potential as a mode to improve scar appearance.
Keywords: NPWT; YAP; caspase3; fibrosis; mechanotransduction; scarring; tissue regeneration; wound healing.
Conflict of interest statement
D.P.O. receives research funding through sponsored research agreement to Brigham and Women’s Hospital from KCI (Kinetic Concepts Inc.).
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

