Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders
- PMID: 36297563
- PMCID: PMC9611586
- DOI: 10.3390/pharmaceutics14102128
Understanding the Multidimensional Effects of Polymorphism, Particle Size and Processing for D-Mannitol Powders
Abstract
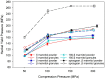
The relevance of the polymorphic form, particle size, and processing of mannitol for the mechanical properties of solid oral dosage forms was examined. Thus, particle and powder properties of spray granulated β D-mannitol, β D-mannitol, and δ D-mannitol were assessed in this study with regards to their manufacturability. D-mannitol is a commonly used excipient in pharmaceutical formulations, especially in oral solid dosage forms, and can be crystallized as three polymorphic forms, of which β is the thermodynamically most stable form and δ is a kinetically stabilized polymorph. A systematic analysis of the powders as starting materials and their respective roller compacted granules is presented to elucidate the multidimensional effects of powder and granules characteristics such as polymorphic form, particle size, and preprocessing on the resulting tablets' mechanical properties. In direct compression and after roller compaction, δ polymorph displayed superior tableting properties over β mannitol, but was outperformed by spray granulated β mannitol. This could be primarily correlated to the higher specific surface area, leading to higher bonding area and more interparticle bonds within the tablet. Hence, it was shown that surface characteristics and preprocessing can prevail over the impact of polymorphism on manufacturability for oral solid dosage forms.
Keywords: direct compression; mannitol; polymorphism; powder characterization; processability; roller compaction; surface area; tabletability.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Caira M.R. Crystalline Polymorphism of Organic Compounds. In: Weber E., editor. Design of Organic Solids. Topics in Current Chemistry. Springer; Berlin/Heidelberg, Germany: 1998.
-
- Hary G. Brittain Polymorphism in Pharmaceutical Solids. 2nd ed. Informa Healthcare USA, Inc.; New York, NY, USA: 2009.
-
- Hilfiker R., von Raumer M. Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2019.
LinkOut - more resources
Full Text Sources

