An Investigation of O-Demethyl Tramadol/Tramadol Ratio for Cytochrome P450 2D6 Phenotyping: The CYTRAM Study
- PMID: 36297612
- PMCID: PMC9611900
- DOI: 10.3390/pharmaceutics14102177
An Investigation of O-Demethyl Tramadol/Tramadol Ratio for Cytochrome P450 2D6 Phenotyping: The CYTRAM Study
Abstract
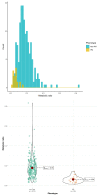
Cytochrome P450 2D6 (CYP2D6) gene polymorphisms influence the exposure to tramadol (T) and its pharmacologically active metabolite, O-demethyl tramadol (O-dT). Tramadol has been considered as a candidate probe drug for CYP2D6 phenotyping. The objective of the CYTRAM study was to investigate the value of plasma O-dT/T ratio for CYP2D6 phenotyping. European adult patients who received IV tramadol after surgery were included. CYP2D6 genotyping was performed and subjects were classified as extensive (EM), intermediate (IM), poor (PM), or ultra-rapid (UM) CYP2D6 metabolizers. Plasma concentrations of tramadol and O-dT were determined at 24 h and 48 h. The relationship between O-dT/T ratio and CYP2D6 phenotype was examined in both a learning and a validation group. Genotype data were obtained in 301 patients, including 23 PM (8%), 117 IM (39%), 154 EM (51%), and 7 UM (2%). Tramadol trough concentrations at 24 h were available in 297 patients. Mean value of O-dT/T ratio was significantly lower in PM than in non-PM individuals (0.061 ± 0.031 versus 0.178 ± 0.09, p < 0.01). However, large overlap was observed in the distributions of O-dT/T ratio between groups. Statistical models based on O-dT/T ratio failed to identify CYP2D6 phenotype with acceptable sensitivity and specificity. Those results suggest that tramadol is not an adequate probe drug for CYP2D6 phenotyping.
Keywords: CYP2D6; pharmacogenetics; pharmacogenomics; tramadol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- The Pharmacogenomics Knowledgebase (PharmGKB) Very Important Pharmacogene: CYP2D6. [(accessed on 27 April 2022)]. Available online: https://www.pharmgkb.org/vip/PA166170264.
-
- Caudle K.E., Sangkuhl K., Whirl-Carrillo M., Swen J.J., Haidar C.E., Klein T.E., Gammal R.S., Relling M.V., Scott S.A., Hertz D.L., et al. Standardizing CYP2D6 Genotype to Phenotype Translation: Consensus Recommendations from the Clinical Pharmacogenetics Implementation Consortium and Dutch Pharmacogenetics Working Group. Clin. Transl. Sci. 2020;13:116–124. doi: 10.1111/cts.12692. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

