Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes
- PMID: 36297746
- PMCID: PMC9612090
- DOI: 10.3390/plants11202722
Recent Updates on Phytoconstituent Alpha-Glucosidase Inhibitors: An Approach towards the Treatment of Type Two Diabetes
Abstract
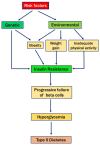
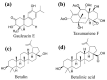
Diabetes is a common metabolic disorder marked by unusually high plasma glucose levels, which can lead to serious consequences such as retinopathy, diabetic neuropathy and cardiovascular disease. One of the most efficient ways to reduce postprandial hyperglycemia (PPHG) in diabetes mellitus, especially insulin-independent diabetes mellitus, is to lower the amount of glucose that is absorbed by inhibiting carbohydrate hydrolyzing enzymes in the digestive system, such as α-glucosidase and α-amylase. α-Glucosidase is a crucial enzyme that catalyzes the final stage of carbohydrate digestion. As a result, α-glucosidase inhibitors can slow D-glucose release from complex carbohydrates and delay glucose absorption, resulting in lower postprandial plasma glucose levels and control of PPHG. Many attempts have been made in recent years to uncover efficient α-glucosidase inhibitors from natural sources to build a physiologic functional diet or lead compound for diabetes treatment. Many phytoconstituent α-glucosidase inhibitors have been identified from plants, including alkaloids, flavonoids, anthocyanins, terpenoids, phenolic compounds, glycosides and others. The current review focuses on the most recent updates on different traditional/medicinal plant extracts and isolated compounds' biological activity that can help in the development of potent therapeutic medications with greater efficacy and safety for the treatment of type 2 diabetes or to avoid PPHG. For this purpose, we provide a summary of the latest scientific literature findings on plant extracts as well as plant-derived bioactive compounds as potential α-glucosidase inhibitors with hypoglycemic effects. Moreover, the review elucidates structural insights of the key drug target, α-glucosidase enzymes, and its interaction with different inhibitors.
Keywords: natural compounds; postprandial hyperglycemia; type 2 diabetes; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


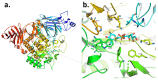

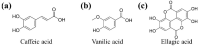
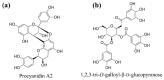
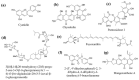
Similar articles
-
α-glucosidase inhibitors from plants: A natural approach to treat diabetes.Pharmacogn Rev. 2011 Jan;5(9):19-29. doi: 10.4103/0973-7847.79096. Pharmacogn Rev. 2011. PMID: 22096315 Free PMC article.
-
Flavonoids as potential agents in the management of type 2 diabetes through the modulation of α-amylase and α-glucosidase activity: a review.Crit Rev Food Sci Nutr. 2022;62(12):3137-3207. doi: 10.1080/10408398.2020.1862755. Epub 2021 Jan 11. Crit Rev Food Sci Nutr. 2022. PMID: 33427491 Review.
-
α-Glucosidase Inhibitory Activity of Selected Malaysian Plants.J Pharm Bioallied Sci. 2017 Jul-Sep;9(3):164-170. doi: 10.4103/jpbs.JPBS_35_17. J Pharm Bioallied Sci. 2017. PMID: 28979070 Free PMC article. Review.
-
Recent Advances in the Development of Alpha-Glucosidase and Alpha-Amylase Inhibitors in Type 2 Diabetes Management: Insights from In silico to In vitro Studies.Curr Drug Targets. 2024 Aug 8. doi: 10.2174/0113894501313365240722100902. Online ahead of print. Curr Drug Targets. 2024. PMID: 39129156
-
An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications.Food Chem Toxicol. 2020 Nov;145:111738. doi: 10.1016/j.fct.2020.111738. Epub 2020 Sep 9. Food Chem Toxicol. 2020. PMID: 32916220 Free PMC article. Review.
Cited by
-
A review on the in vitro and in vivo screening of α-glucosidase inhibitors.Heliyon. 2024 Sep 8;10(18):e37467. doi: 10.1016/j.heliyon.2024.e37467. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309836 Free PMC article. Review.
-
In Vitro and In Vivo Evaluation for Antioxidant and Anti-Diabetic Properties of Cyperus rotundus L. Kombucha.Foods. 2023 Nov 8;12(22):4059. doi: 10.3390/foods12224059. Foods. 2023. PMID: 38002116 Free PMC article.
-
Seaweeds as Nutraceutical Elements and Drugs for Diabetes Mellitus: Future Perspectives.Mar Drugs. 2024 Apr 10;22(4):168. doi: 10.3390/md22040168. Mar Drugs. 2024. PMID: 38667785 Free PMC article. Review.
-
In vitro and in silico pharmaco-nutritional assessments of some lesser-known Nigerian nuts: Persea americana, Tetracarpidium conophorum, and Terminalia catappa.PLoS One. 2025 Apr 9;20(4):e0319756. doi: 10.1371/journal.pone.0319756. eCollection 2025. PLoS One. 2025. PMID: 40202972 Free PMC article.
-
Butyrate's (a short-chain fatty acid) microbial synthesis, absorption, and preventive roles against colorectal and lung cancer.Arch Microbiol. 2024 Mar 4;206(4):137. doi: 10.1007/s00203-024-03834-7. Arch Microbiol. 2024. PMID: 38436734 Review.
References
-
- IDF . International Diabetes Federation IDF Diabetes Atlas. 10th ed. IDF; Brussels, Belgium: 2021. [(accessed on 1 August 2022)]. Available online: https://diabetesatlas.org/idfawp/resource-files/2021/07/IDF_Atlas_10th_E....
-
- Kumar Tripathi B., Srivastava A.K. Diabetes mellitus: Complications and therapeutics RA130. Med. Sci. Monit. 2006;12:130–147. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

