Integrated Omic Approaches Reveal Molecular Mechanisms of Tolerance during Soybean and Meloidogyne incognita Interactions
- PMID: 36297768
- PMCID: PMC9612212
- DOI: 10.3390/plants11202744
Integrated Omic Approaches Reveal Molecular Mechanisms of Tolerance during Soybean and Meloidogyne incognita Interactions
Abstract
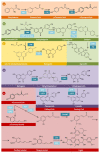
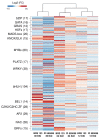
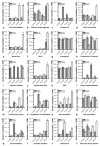
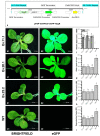
The root-knot nematode (RKN), Meloidogyne incognita, is a devastating soybean pathogen worldwide. The use of resistant cultivars is the most effective method to prevent economic losses caused by RKNs. To elucidate the mechanisms involved in resistance to RKN, we determined the proteome and transcriptome profiles from roots of susceptible (BRS133) and highly tolerant (PI 595099) Glycine max genotypes 4, 12, and 30 days after RKN infestation. After in silico analysis, we described major defense molecules and mechanisms considered constitutive responses to nematode infestation, such as mTOR, PI3K-Akt, relaxin, and thermogenesis. The integrated data allowed us to identify protein families and metabolic pathways exclusively regulated in tolerant soybean genotypes. Among them, we highlighted the phenylpropanoid pathway as an early, robust, and systemic defense process capable of controlling M. incognita reproduction. Associated with this metabolic pathway, 29 differentially expressed genes encoding 11 different enzymes were identified, mainly from the flavonoid and derivative pathways. Based on differential expression in transcriptomic and proteomic data, as well as in the expression profile by RT-qPCR, and previous studies, we selected and overexpressed the GmPR10 gene in transgenic tobacco to assess its protective effect against M. incognita. Transgenic plants of the T2 generation showed up to 58% reduction in the M. incognita reproduction factor. Finally, data suggest that GmPR10 overexpression can be effective against the plant parasitic nematode M. incognita, but its mechanism of action remains unclear. These findings will help develop new engineered soybean genotypes with higher performance in response to RKN infections.
Keywords: differential expression; phenylpropanoids; proteome; root-knot nematode; transcriptome.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures




Similar articles
-
Overexpression of the GmEXPA1 gene reduces plant susceptibility to Meloidogyne incognita.Plant Cell Rep. 2023 Jan;42(1):137-152. doi: 10.1007/s00299-022-02941-3. Epub 2022 Nov 8. Plant Cell Rep. 2023. PMID: 36348064
-
Transcriptome analysis of resistant and susceptible alfalfa cultivars infected with root-knot nematode Meloidogyne incognita.PLoS One. 2015 Mar 30;10(3):e0123157. doi: 10.1371/journal.pone.0123157. eCollection 2015. PLoS One. 2015. PMID: 25822722 Free PMC article.
-
Transcriptome analysis of root-knot nematode (Meloidogyne incognita)-resistant and susceptible sweetpotato cultivars.Planta. 2019 Feb;249(2):431-444. doi: 10.1007/s00425-018-3001-z. Epub 2018 Sep 19. Planta. 2019. PMID: 30232599
-
Transcriptome analysis of resistant and susceptible alfalfa cultivars infected with root-knot nematode Meloidogyne incognita.PLoS One. 2015 Feb 24;10(2):e0118269. doi: 10.1371/journal.pone.0118269. eCollection 2015. PLoS One. 2015. PMID: 25710378 Free PMC article.
-
Transcriptome analysis of resistant and susceptible tobacco (Nicotiana tabacum) in response to root-knot nematode Meloidogyne incognita infection.Biochem Biophys Res Commun. 2017 Jan 22;482(4):1114-1121. doi: 10.1016/j.bbrc.2016.11.167. Epub 2016 Dec 1. Biochem Biophys Res Commun. 2017. PMID: 27914810
Cited by
-
Soybean genomics research community strategic plan: A vision for 2024-2028.Plant Genome. 2024 Dec;17(4):e20516. doi: 10.1002/tpg2.20516. Epub 2024 Nov 21. Plant Genome. 2024. PMID: 39572930 Free PMC article.
-
Combating Root-Knot Nematodes (Meloidogyne spp.): From Molecular Mechanisms to Resistant Crops.Plants (Basel). 2025 Apr 27;14(9):1321. doi: 10.3390/plants14091321. Plants (Basel). 2025. PMID: 40364350 Free PMC article. Review.
-
Growth, Physiological, and Biochemical Variations in Tomatoes after Infection with Different Density Levels of Meloidogyne enterolobii.Plants (Basel). 2024 Jan 18;13(2):293. doi: 10.3390/plants13020293. Plants (Basel). 2024. PMID: 38256846 Free PMC article.
-
Biochemical Defence of Plants against Parasitic Nematodes.Plants (Basel). 2024 Oct 8;13(19):2813. doi: 10.3390/plants13192813. Plants (Basel). 2024. PMID: 39409684 Free PMC article. Review.
-
Transcriptome analysis of two tobacco varieties with contrast resistance to Meloidogyne incognita in response to PVY MSNR infection.Front Plant Sci. 2023 Aug 28;14:1213494. doi: 10.3389/fpls.2023.1213494. eCollection 2023. Front Plant Sci. 2023. PMID: 37701805 Free PMC article.
References
-
- Pimentel D., Patzek T.W. Ethanol production using corn, switchgrass, and wood; biodiesel production using soybean and sunflower. Nat. Resour. Res. 2005;14:65–76. doi: 10.1007/s11053-005-4679-8. - DOI
-
- Wilson R.F. Soybean: Market driven research needs. In: Stacey G., editor. Genetics and Genomics of Soybean. Springer; New York, NY, USA: 2008. pp. 3–15.
-
- Peng D., Gatschke J. The Statistics Portal for Market Data, Market Research and Market Studies. Statista; Hamburg, Germany: 2021.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

