Nonhuman Primates Are Protected against Marburg Virus Disease by Vaccination with a Vesicular Stomatitis Virus Vector-Based Vaccine Prepared under Conditions to Allow Advancement to Human Clinical Trials
- PMID: 36298451
- PMCID: PMC9610558
- DOI: 10.3390/vaccines10101582
Nonhuman Primates Are Protected against Marburg Virus Disease by Vaccination with a Vesicular Stomatitis Virus Vector-Based Vaccine Prepared under Conditions to Allow Advancement to Human Clinical Trials
Abstract
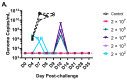
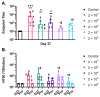
Vaccines are needed to disrupt or prevent continued outbreaks of filoviruses in humans across Western and Central Africa, including outbreaks of Marburg virus (MARV). As part of a filovirus vaccine product development plan, it is important to investigate dose response early in preclinical development to identify the dose range that may be optimal for safety, immunogenicity, and efficacy, and perhaps demonstrate that using lower doses is feasible, which will improve product access. To determine the efficacious dose range for a manufacturing-ready live recombinant vesicular stomatitis virus vaccine vector (rVSV∆G-MARV-GP) encoding the MARV glycoprotein (GP), a dose-range study was conducted in cynomolgus macaques. Results showed that a single intramuscular injection with as little as 200 plaque-forming units (PFUs) was 100% efficacious against lethality and prevented development of viremia and clinical pathologies associated with MARV Angola infection. Across the vaccine doses tested, there was nearly a 2000-fold range of anti-MARV glycoprotein (GP) serum IgG titers with seroconversion detectable even at the lowest doses. Virus-neutralizing serum antibodies also were detected in animals vaccinated with the higher vaccine doses indicating that vaccination induced functional antibodies, but that the assay was a less sensitive indicator of seroconversion. Collectively, the data indicates that a relatively wide range of anti-GP serum IgG titers are observed in animals that are protected from disease implying that seroconversion is positively associated with efficacy, but that more extensive immunologic analyses on samples collected from our study as well as future preclinical studies will be valuable in identifying additional immune responses correlated with protection that can serve as markers to monitor in human trials needed to generate data that can support vaccine licensure in the future.
Keywords: Marburg virus vaccine; emerging infectious disease; filoviruses; vaccine vector; vesicular stomatitis virus.
Conflict of interest statement
C.L.C., C.L.P., M.Y., E.S., J.W.C. and M.B.F. have filed a provisional patent application on the Marburg virus vaccine candidate. The authors declare no other conflict of interest.
Figures


References
-
- WHO Ebola Virus Disease—Democratic Republic of the Congo. [(accessed on 8 July 2022)]. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON377.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

