The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice
- PMID: 36298468
- PMCID: PMC9612271
- DOI: 10.3390/vaccines10101603
The Deletion of US3 Gene of Pseudorabies Virus (PRV) ΔgE/TK Strain Induces Increased Immunogenicity in Mice
Abstract
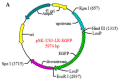
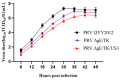
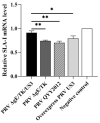
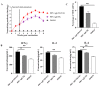
Re-emerging pseudorabies (PR) caused by pseudorabies virus (PRV) variant has been prevailing among immunized herds in China since 2011, indicating that commercially available PR vaccine strains couldn't provide complete protection against novel, epidemic PRV variant. Before this study, a gE/TK-gene-deleted virus (PRV ΔgE/TK) was constructed from PRV QYY2012 variant through homologous recombination and Cre/LoxP system. Here, PRV ΔgE/TK/US3 strain was generated by deleting US3 gene based on PRV ΔgE/TK strain using the same method. The growth characteristics of PRV ΔgE/TK/US3 were analogous to that of PRV ΔgE/TK. Moreover, the deletion of US3 gene could promote apoptosis, upregulate the level of swine leukocyte antigen class I molecule (SLA-I) in vitro, and relieve inflammatory response in inoculated BALB/c mice. Subsequently, the safety and immunogenicity of PRV ΔgE/TK/US3 was evaluated as a vaccine candidate in mice. The results revealed that PRV ΔgE/TK/US3 was safe for mice, and mice vaccinated with PRV ΔgE/TK/US3 could induce a higher level of PRV-specific neutralizing antibodies and cytokines, including IFN-γ, IL-2 and IL-4, also higher level of CD8+ CD69+ Tissue-Resident Memory T cells (TRM). The results show that the deletion of US3 gene of PRV ΔgE/TK strain could induce increased immunogenicity, indicating that the PRV ΔgE/TK/US3 strain is a promising vaccine candidate for preventing and controlling of the epidemic PR in China.
Keywords: PRV ΔgE/TK/US3; US3 gene; immunogenicity; pseudorabies virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

