Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents
- PMID: 36298510
- PMCID: PMC9609361
- DOI: 10.3390/vaccines10101646
Immunogenicity of a Fractional Dose of mRNA BNT162b2 COVID-19 Vaccine for Primary Series and Booster Vaccination among Healthy Adolescents
Abstract
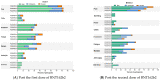
Primary series vaccination with BNT162b2 followed by a booster 5 months later has been recommended for healthy adolescents. We aimed to describe the immunogenicity in a fractional dose of BNT162b2. Adolescents aged 12-18 years were randomized into six arms for primary series administration: 3wPZ30/30 (reference group), 3wPZ30/20, 3wPZ20/20, 6wPZ30/30, 6wPZ30/20, and 6wPZ20/20 μg. A booster was given at 5 months after the second dose using either 10 or 15 μg of BNT162b2. Immunogenicity following vaccination was determined by IgG against receptor-binding domain (anti-S-RBD IgG; BAU/mL), surrogate virus neutralization test (sVNT; %inhibition) and pseudovirus neutralization (pVNT;ID50) against Omicron. Non-inferiority criteria were defined as a lower boundary of the geometric mean ratio (GMR) being greater than 0.67. From September to October 2021, 118 adolescents with a median age (IQR) of 14.9 years (13.9-16.7) were enrolled. Fourteen days after the primary series, the geometric means (GMs) of anti-S-RBD IgG (BAU/mL) were 3090 (95% CI 2761-3460) in 3wPZ30/30. The GMRs of anti-S-RBD were: 0.80 (95% CI 0.67-0.97) in 3wPZ30/20; 1.00 (95% CI 0.83-1.20) in 3wPZ20/20; 1.37 (95% CI 1.13-1.65) in 6wPZ30/30; 1.24 (95% CI 1.02-1.50) in 6wPZ30/20; and 1.36 (1.13-1.64) in 6wPZ20/20. After a booster dose with 15 μg (n = 24) of BNT162b2, sVNT and pVNT against Omicron variant were 91.6 (95% CI 88.4-94.9) and 331 (95% CI 221-495), respectively. In the group that received 10 μg of BNT162b2 (n = 25), sVNT was 85.6 (95% CI 80.0-91.6) and pVNT was 397 (95% CI 267-590). Healthy adolescents had good immune responses to the fractional dose regimen of BNT162b2 and this may be considered as an alternative option.
Keywords: BNT162b2; SARS-CoV-2 vaccine; adolescents; anti-SARS-CoV-2; booster dose; fractional dose; neutralizing antibody titer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization WHO Coronavirus Disease (COVID-19) Dashboard. 2022. [(accessed on 28 August 2022)]. Available online: https://covid19.who.int/
-
- Department of Disease Control, Ministry of Public Health (Thailand) COVID-19 Dash Board. [(accessed on 28 August 2022)];2022 Available online: https://ddc.moph.go.th/covid19-dashboard/?dashboard=select-trend-line.
-
- World Health Organization COVID-19 Weekly Epidemiological Update Edition 42. [(accessed on 14 August 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- World Health Organization COVID-19 Weekly Epidemiological Update Edition 74. [(accessed on 14 August 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

