Recurrent and De Novo Toxoplasmosis Retinochoroiditis following Coronavirus Disease 2019 Infection or Vaccination
- PMID: 36298557
- PMCID: PMC9609888
- DOI: 10.3390/vaccines10101692
Recurrent and De Novo Toxoplasmosis Retinochoroiditis following Coronavirus Disease 2019 Infection or Vaccination
Abstract
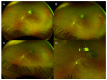
This study reports three cases of toxoplasmosis retinochoroiditis following coronavirus disease 2019 (COVID-19) infection or vaccination from the national Canadian COVID-19 Eye Registry between December 2020 and September 2021. A 56-year-old male presented 15 days after a positive COVID-19 test with toxoplasmosis retinochoroiditis. He later relapsed 8 days following a first Pfizer-BioNTech vaccine dose. Two patients presented with toxoplasmosis retinochoroiditis following COVID-19 vaccination: A 58-year-old female presenting 4 days following a first Pfizer-BioNTech vaccine dose with anterior uveitis and a posterior pole lesion discovered 3 months later and a 39-year-old female presenting 17 days after a first Moderna vaccine dose. Resolution was achieved with oral clindamycin, oral trimethoprim/sulfamethoxazole, and topical prednisolone acetate 1%. Patients were offered prophylactic trimethoprim/sulfamethoxazole for subsequent doses without relapse. Following COVID-19 infection or vaccination, patients may be at risk for toxoplasmosis retinochoroiditis. Prophylactic antibiotics for future doses may be offered to patients with known ocular toxoplasmosis to prevent recurrence.
Keywords: COVID-19 vaccination; SARS-CoV-2; antibiotic prophylaxis; coronavirus disease 2019; inflammation; mRNA vaccine; ophthalmic adverse events; toxoplasmosis retinochoroiditis; vaccination.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

