Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach
- PMID: 36298612
- PMCID: PMC9608090
- DOI: 10.3390/vaccines10101747
Identifying Modifiable Predictors of COVID-19 Vaccine Side Effects: A Machine Learning Approach
Abstract
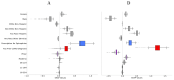
Side effects of COVID-19 or other vaccinations may affect an individual's safety, ability to work or care for self or others, and/or willingness to be vaccinated. Identifying modifiable factors that influence these side effects may increase the number of people vaccinated. In this observational study, data were from individuals who received an mRNA COVID-19 vaccine between December 2020 and April 2021 and responded to at least one post-vaccination symptoms survey that was sent daily for three days after each vaccination. We excluded those with a COVID-19 diagnosis or positive SARS-CoV2 test within one week after their vaccination because of the overlap of symptoms. We used machine learning techniques to analyze the data after the first vaccination. Data from 50,484 individuals (73% female, 18 to 95 years old) were included in the primary analysis. Demographics, history of an epinephrine autoinjector prescription, allergy history category (e.g., food, vaccine, medication, insect sting, seasonal), prior COVID-19 diagnosis or positive test, and vaccine manufacturer were identified as factors associated with allergic and non-allergic side effects; vaccination time 6:00-10:59 was associated with more non-allergic side effects. Randomized controlled trials should be conducted to quantify the relative effect of modifiable factors, such as time of vaccination.
Keywords: COVID-19; allergy; machine learning; model explanation; side effects; time-of-day-effects; vaccination.
Conflict of interest statement
S.A.; D.H.; K.H.; S.S.M.: None. G.K.R.: Trial support from Leonard Meron Bioscience, Consulting for Teradyne Inc; outside the submitted work. Member of the DHHS OI guidelines. K.G.B.: Grant support from Phadia Ab (Thermo Fisher Scientific); personal fees for legal case review from Weekley Shulte Valdes Murman Tonelli, Piedmont Liability Trust, Vasios Kelly and Strollo PA, and Publix Supermarkets; and royalties from UpToDate, all outside the submitted work. E.S.S.: a writer for Up To Date; outside the submitted work. W.W.: Consultancy for National Sleep Foundation, outside the submitted work. E.B.K.: Consulting for American Academy of Sleep Medicine Foundation, Circadian Therapeutics National Sleep Foundation, Sleep Research Society Foundation, Yale University Press; travel support from European Biological Rhythms Society; partner owns Chronsulting. All outside the submitted work.
Figures





References
-
- CDC COVID-19 Vaccines Work. [(accessed on 1 August 2022)];2021 Available online: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/effectiveness/work.html.
-
- Coronavirus (COVID-19) Vaccinations. 2022. [(accessed on 1 August 2022)]. Available online: https://ourworldindata.org/covid-vaccinations.
-
- Shenoy E.S., Wickner P.G., West L.R., Banerji A., Blumenthal K.G., Centi A.J., Gottlieb A., Hashimoto D.M., Kim E., Kim M., et al. Symptom monitoring after coronavirus disease 2019 (COVID-19) vaccination in a large integrated healthcare system: Separating symptoms from severe acute respiratory coronavirus virus 2 (SARS-CoV-2) infection. Infect. Control Hosp. Epidemiol. 2021:1–8. doi: 10.1017/ice.2021.449. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

