Post-Vaccination Neutralization Responses to Omicron Sub-Variants
- PMID: 36298622
- PMCID: PMC9607453
- DOI: 10.3390/vaccines10101757
Post-Vaccination Neutralization Responses to Omicron Sub-Variants
Abstract
Background: The emergence of the Omicron variant (B.1.1.529), which correlated with dramatic losses in cross-neutralization capacity of post-vaccination sera, raised concerns about the effectiveness of COVID-19 vaccines against infection and disease. Several clinically relevant sub-variants subsequently emerged rapidly.
Methods: We evaluated published and pre-print studies reporting sub-variant specific reductions in cross-neutralization compared to the prototype strain of SARS-CoV-2 and between sub-variants. Median fold-reduction across studies was calculated by sub-variant and vaccine platform.
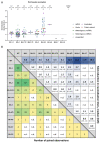
Results: Among 178 studies with post-vaccination data, after primary vaccination the sub-variant specific fold-reduction in neutralization capacity compared to the prototype antigen varied widely, from median 4.2-fold for BA.3 to 40.1-fold for BA.2.75; in boosted participants fold-reduction was similar for most sub-variants (5.3-fold to 7.0-fold); however, a more pronounced fold-change was observed for sub-variants related to BA.4 and BA.5 (10.4-fold to 14.2-fold). Relative to BA.1, the other Omicron sub-variants had similar neutralization capacity post-primary vaccination (range median 0.8-fold to 1.1-fold) and post-booster (0.9-fold to 1.4-fold) except for BA.4/5-related sub-variants which was higher (2.1-fold to 2.7-fold). Omicron sub-variant-specific responder rates were low post-primary vaccination (range median 28.0% to 65.9%) compared to the prototype (median 100%) but improved post-booster (range median 73.3% to 100%).
Conclusions: Fold-reductions in neutralization titers were comparable post-booster except for sub-variants related to BA.4 and BA.5, which had higher fold-reduction. Assessment after primary vaccination was not possible because of overall poor neutralization responses causing extreme heterogeneity. Considering large fold-decreases in neutralization titers relative to the parental strain for all Omicron sub-variants, vaccine effectiveness is very likely to be reduced against all Omicron sub-variants, and probably more so against variants related to BA.4 or BA.5.
Keywords: COVID-19 vaccine; Omicron; SARS-CoV-2; neutralization; sub-variant.
Conflict of interest statement
M.M.H. reports research grants from Pfizer for unrelated work; M.D.K. reports research grants from Pfizer and Merck and consultancy fees from Merck, all for unrelated work; N.B.-Z. reports research grants from Merck, from Johnson & Johnson and from Serum Institute of India, all for unrelated work; and consulting fees from Merck for unrelated work.
Figures

References
-
- WHO Weekly Epidemiological Update on COVID-19–29 June 2022. 2022. [(accessed on 1 October 2022)]. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
-
- Edara V.V., Manning K.E., Ellis M., Lai L., Moore K.M., Foster S.L., Floyd K., Daves-Gardner M.E., Mantus G., Nyhoff L.E. mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 omicron variant. Cell Rep. Med. 2022;3:100529. doi: 10.1016/j.xcrm.2022.100529. - DOI - PMC - PubMed
-
- Cheng S.M.S., Mok C.K.P., Leung Y.W.Y., Ng S.S., Chan K.C.K., Ko F.W., Chen C., Yio K., Lam B.H.S., Lau E.H.Y. Neutralizing antibodies against the SARS-CoV-2 Omicron variant BA.1 following homologous and heterologous CoronaVac or BNT162b2 vaccination. Nat. Med. 2022;28:486–489. doi: 10.1038/s41591-022-01704-7. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

