Chimeric Virus-like Particles Co-Displaying Hemagglutinin Stem and the C-Terminal Fragment of DnaK Confer Heterologous Influenza Protection in Mice
- PMID: 36298664
- PMCID: PMC9610613
- DOI: 10.3390/v14102109
Chimeric Virus-like Particles Co-Displaying Hemagglutinin Stem and the C-Terminal Fragment of DnaK Confer Heterologous Influenza Protection in Mice
Abstract
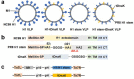
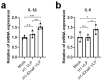
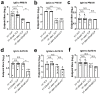
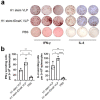
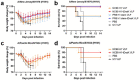
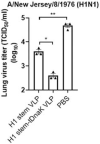
Influenza virus hemagglutinin (HA) stem is currently regarded as an extremely promising immunogen for designing universal influenza vaccines. The appropriate antigen-presenting vaccine vector would be conducive to increasing the immunogenicity of the HA stem antigen. In this study, we generated chimeric virus-like particles (cVLPs) co-displaying the truncated C-terminal of DnaK from Escherichia coli and H1 stem or full-length H1 antigen using the baculovirus expression system. Transmission electronic micrography revealed the expression and presentation of H1 stem antigens on the surface of VLPs. Vaccinations of mice with the H1 stem cVLPs induced H1-specific immune responses and provided heterologous immune protection in vivo, which was more effective than vaccinations with VLPs displaying H1 stem alone in protecting mice against weight loss as well as increasing survival rates after lethal influenza viral challenge. The results indicate that the incorporation of the truncated C-terminal of DnaK as an adjuvant protein into the cVLPs significantly enhances the H1-specific immunity and immune protection. We have explicitly identified the VLP platform as an effective way of expressing HA stem antigen and revealed that chimeric VLP is an vaccine vector for developing HA stem-based universal influenza vaccines.
Keywords: DnaK; hemagglutinin stem; influenza virus; universal vaccine; virus-like particle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

