Lessons from the Cerebrospinal Fluid Analysis of HTLV-1-Infected Individuals: Biomarkers of Inflammation for HAM/TSP Development
- PMID: 36298702
- PMCID: PMC9609689
- DOI: 10.3390/v14102146
Lessons from the Cerebrospinal Fluid Analysis of HTLV-1-Infected Individuals: Biomarkers of Inflammation for HAM/TSP Development
Abstract
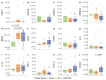
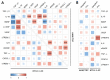
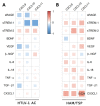
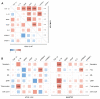
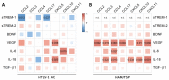
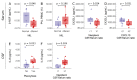
HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a neurodegenerative disease that leads to motor impairment due to a chronic inflammatory process in the central nervous system (CNS). However, the HAM/TSP pathogenesis is not completely clear, and biomarkers to define the disease prognosis are still necessary. Thus, we aimed to identify biomarkers for HAM/TSP and potential mechanisms involved in disease development. To that end, the concentrations of VILIP-1, BDNF, VEGF, β-NGF, TGF-β1, fractalkine/CX3CL1, IL-6, IL-18, and TNF-α, and the soluble forms of TREM-1, TREM-2, and RAGE, were assessed using a multiplex bead-based immunoassay in paired cerebrospinal fluid (CSF) and serum samples from HAM/TSP patients (n = 20), asymptomatic HTLV-1 carriers (AC) (n = 13), and HTLV-1-seronegative individuals (n = 9), with the results analyzed according to the speed of HAM/TSP progression. HAM/TSP patients had elevated fractalkine in the serum but not in the CSF, particularly those with low neuroinflammatory activity (CSF/serum ratio of neopterin <1 and of CXCL10 < 2). HAM/TSP patients with normal CSF levels of neurofilament light chain (NfL) showed elevated β-NGF in serum, and serum BDNF levels were increased in HTLV-1-infected individuals, particularly in HTLV-1 AC. Both HTLV-1 AC and HAM/TSP patients had lower TGF-β1 levels in CSF compared to uninfected individuals, and HAM/TSP patients with active CNS inflammation showed higher CSF levels of IL-18, which correlated with markers of inflammation, neuronal death, and blood−brain-barrier permeability. Although none of the factors evaluated were associated with the speed of HAM/TSP progression, reduced TGF-β1 levels in CSF suggest that suppressive responses to control subclinical and/or active neurodegeneration are impaired, while increased CSF IL-18 indicates the involvement of inflammasome-mediated mechanisms in HAM/TSP development.
Keywords: HAM/TSP; HTLV-1; IL-18; biomarkers; cerebrospinal fluid; inflammasome; neurodegeneration.
Conflict of interest statement
The authors declare no conflict of interest, and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

