A Transcriptomics-Based Bioinformatics Approach for Identification and In Vitro Screening of FDA-Approved Drugs for Repurposing against Dengue Virus-2
- PMID: 36298705
- PMCID: PMC9609047
- DOI: 10.3390/v14102150
A Transcriptomics-Based Bioinformatics Approach for Identification and In Vitro Screening of FDA-Approved Drugs for Repurposing against Dengue Virus-2
Abstract
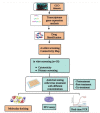
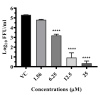
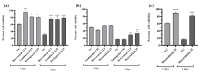
The rising incidence of dengue virus (DENV) infections in the tropical and sub-tropical regions of the world emphasizes the need to identify effective therapeutic drugs against the disease. Repurposing of drugs has emerged as a novel concept to combat pathogens. In this study, we employed a transcriptomics-based bioinformatics approach for drug identification against DENV. Gene expression omnibus datasets from patients with different grades of dengue disease severity and healthy controls were used to identify differentially expressed genes in dengue cases, which were then applied to the query tool of Connectivity Map to identify the inverse gene-disease-drug relationship. A total of sixteen identified drugs were investigated for their prophylactic, virucidal, and therapeutic effects against DENV. Focus-forming unit assay and quantitative RT-PCR were used to evaluate the antiviral activity. Results revealed that five compounds, viz., resveratrol, doxorubicin, lomibuvir, elvitegravir, and enalaprilat, have significant anti-DENV activity. Further, molecular docking studies showed that these drugs can interact with a variety of protein targets of DENV, including the glycoprotein, the NS5 RdRp, NS2B-NS3 protease, and NS5 methyltransferase The in vitro and in silico results, therefore, reveal that these drugs have the ability to decrease DENV-2 production, suggesting that these drugs or their derivatives could be attempted as therapeutic agents against DENV infections.
Keywords: DENV-2; FDA-approved drugs; antiviral therapy; dengue fever; repurposing drugs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Dengue and Severe Dengue. [(accessed on 17 January 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue.
-
- Murugesan A., Manoharan M. Dengue Virus. Emerg. Reemerging Viral Pathog. 2020;1:281–359. doi: 10.1016/B978-0-12-819400-3.00016-8. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

