Performing under Pressure: Insights into the Diagnostic Testing Burden at a UK National Health Service Clinical Virology Laboratory during the SARS-CoV-2 Pandemic
- PMID: 36298788
- PMCID: PMC9609557
- DOI: 10.3390/v14102233
Performing under Pressure: Insights into the Diagnostic Testing Burden at a UK National Health Service Clinical Virology Laboratory during the SARS-CoV-2 Pandemic
Abstract
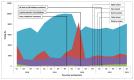
UK National Health Service (NHS) Clinical Virology Departments provide a repertoire of tests on clinical samples to detect the presence of viral genomic material or host immune responses to viral infection. In December 2019, a novel coronavirus (SARS-CoV-2) emerged which quickly developed into a global pandemic; NHS laboratories responded rapidly to upscale their testing capabilities. To date, there is little information on the impact of increased SARS-CoV-2 screening on non-SARS-CoV-2 testing within NHS laboratories. This report details the virology test requests received by the Leicester-based NHS Virology laboratory from January 2018 to May 2022. Data show that in spite of a dramatic increase in screening, along with multiple logistic and staffing issues, the Leicester Virology Department was mostly able to maintain the same level of service for non-respiratory virus testing while meeting the new increase in SARS-CoV-2 testing.
Keywords: COVID-19; SARS-CoV-2; burden; diagnostics; non-SARS-CoV-2; pandemic; routine; testing.
Conflict of interest statement
There are no declarations of competing interests to be declared by the authors.
Figures

References
-
- The Royal College of Pathologists Pathology Facts and Figures. [(accessed on 16 August 2022)]. Available online: https://www.rcpath.org/discover-pathology/news/fact-sheets/pathology-fac....
-
- National Pathology Programme Digital First: Clinical Transformation Through Pathology Innovation. [(accessed on 16 August 2022)]. Available online: https://www.england.nhs.uk/wp-content/uploads/2014/02/pathol-dig-first.pdf.
-
- Institute for Government Analysis Timeline of UK Coronavirus Lockdowns, March 2020 to March 2021. [(accessed on 16 August 2022)]. Available online: https://www.instituteforgovernment.org.uk/sites/default/files/timeline-l....
-
- Department of Health & Social Care Guidance: COVID-19 Testing Data: Methodology Note. [(accessed on 16 August 2022)]; Available online: https://www.gov.uk/government/publications/coronavirus-COVID-19-testing-....
-
- Department of Health & Social Care Three Lighthouse Laboratories Begin Testing for COVID-19. [(accessed on 16 August 2022)]; Available online: https://www.gov.uk/government/news/three-lighthouse-laboratories-begin-t....
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

