SARS-CoV-2 Pre-Exposure Prophylaxis with Sotrovimab and Tixagevimab/Cilgavimab in Immunocompromised Patients-A Single-Center Experience
- PMID: 36298832
- PMCID: PMC9607212
- DOI: 10.3390/v14102278
SARS-CoV-2 Pre-Exposure Prophylaxis with Sotrovimab and Tixagevimab/Cilgavimab in Immunocompromised Patients-A Single-Center Experience
Abstract
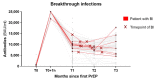
Immunocompromised patients experience reduced vaccine effectiveness and are at higher risk for coronavirus disease 19 (COVID-19) death. Pre-exposure prophylaxis (PrEP) aims to protect these patients. So far, only tixagevimab/cilgavimab is authorized for use as PrEP. This paper aims to provide real-world data on the use of tixagevimab/cilgavimab and sotrovimab as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) PrEP in immunocompromised patients, comparing the evolution of antibody levels and reporting the incidence of breakthrough infections. A retrospective, single-center analysis was conducted including 132 immunocompromised patients with inadequate vaccine response, who received COVID-PrEP at our clinic between January and June 2022. Initially, 95 patients received sotrovimab while 37 patients received tixagevimab/cilgavimab. Antibody levels after first PrEP with sotrovimab remain high for several months after infusion (median 10,058 and 7235 BAU/mL after 1 and 3 months, respectively), with higher titers than after tixagevimab/cilgavimab injection even 3 months later (7235 vs. 1647 BAU/mL, p = 0.0007). Overall, breakthrough infections were rare (13/132, 10%) when compared to overall infection rates during this period (over 30% of the Austrian population), with mild disease course and rapid viral clearance (median 10 days). Sotrovimab may be an additional option for SARS-CoV-2 PrEP.
Keywords: SARS-CoV-2 PrEP; immunocompromised patients; pre-exposure prophylaxis; sotrovimab; tixagevimab/cilgavimab.
Conflict of interest statement
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU) 2021. [(accessed on 4 July 2021)]. Available online: https://coronavirus.jhu.edu/map.html.
-
- Plumb I.D., Feldstein L.R., Barkley E., Posner A.B., Bregman H.S., Hagen M.B., Gerhart J.L. Effectiveness of COVID-19 mRNA Vaccination in Preventing COVID-19–Associated Hospitalization Among Adults with Previous SARS-CoV-2 Infection—United States, June 2021–February 2022. MMWR Morb. Mortal Wkly. Rep. 2022;71:549–555. doi: 10.15585/mmwr.mm7115e2. - DOI - PMC - PubMed
-
- Bernal J.L., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., Simmons R., Cottrell S., Roberts R., O’Doherty M., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on COVID-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

