Recombinant Human Cytomegalovirus Expressing an Analog-Sensitive Kinase pUL97 as Novel Tool for Functional Analyses
- PMID: 36298840
- PMCID: PMC9610083
- DOI: 10.3390/v14102285
Recombinant Human Cytomegalovirus Expressing an Analog-Sensitive Kinase pUL97 as Novel Tool for Functional Analyses
Abstract
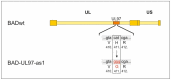
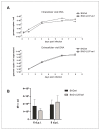
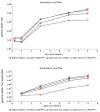
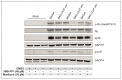
The human cytomegalovirus (HCMV) is a member of the beta-herpesvirus family and inflicts life-long latent infections in its hosts. HCMV has been shown to manipulate and dysregulate many cellular processes. One major interactor with the cellular host is the viral kinase pUL97. The UL97 gene is essential for viral replication, and kinase-deficient mutants of pUL97 display a severe replication defect. Recently, another group established an analog-sensitive version of the pUL97 protein. This mutant kinase can be treated with a non-hydrolysable ATP analog, thereby inhibiting its kinase function. This process is reversible by removing the ATP analog by media change. We introduced this mutant version of the pUL97 protein into the laboratory strain Ad169 of HCMV, BADwt, creating a BAD-UL97-as1 viral mutant. This mutant virus replicated normally in infected cells in the absence of the ATP analog and maintained its ability to phosphorylate its cellular substrates. However, when treated with the ATP analog, BAD-UL97-as1 displayed a defect in the production of intra- and extracellular viral DNA and in the production of viral progeny. Furthermore, in the presence of 3MB-PP1, a well-established substrate of pUL97 was no longer hyperphosphorylated. This effect was detectable as early as 4 h post treatment, which allows for studies on pUL97 without the complication of low viral titers. Nevertheless, we observed off-target effects of 3MB-PP1 on several cellular processes, which should be considered with this approach.
Keywords: BACmid-derived recombinant HCMV; analog-sensitive pUL97 variant; functional analyses; human cytomegalovirus (HCMV); pUL97-specific inhibitors; protein kinase pUL97; viral kinase activity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Highly Conserved Interaction Profiles between Clinically Relevant Mutants of the Cytomegalovirus CDK-like Kinase pUL97 and Human Cyclins: Functional Significance of Cyclin H.Int J Mol Sci. 2022 Oct 5;23(19):11814. doi: 10.3390/ijms231911814. Int J Mol Sci. 2022. PMID: 36233116 Free PMC article.
-
Inhibitors of human cytomegalovirus replication drastically reduce the activity of the viral protein kinase pUL97.J Gen Virol. 2001 Jun;82(Pt 6):1439-1450. doi: 10.1099/0022-1317-82-6-1439. J Gen Virol. 2001. PMID: 11369889
-
Comparison between human cytomegalovirus pUL97 and murine cytomegalovirus (MCMV) pM97 expressed by MCMV and vaccinia virus: pM97 does not confer ganciclovir sensitivity.J Virol. 2000 Nov;74(22):10729-36. doi: 10.1128/jvi.74.22.10729-10736.2000. J Virol. 2000. PMID: 11044117 Free PMC article.
-
Regulatory roles of protein kinases in cytomegalovirus replication.Adv Virus Res. 2011;80:69-101. doi: 10.1016/B978-0-12-385987-7.00004-X. Adv Virus Res. 2011. PMID: 21762822 Review.
-
Understanding the Cytomegalovirus Cyclin-Dependent Kinase Ortholog pUL97 as a Multifaceted Regulator and an Antiviral Drug Target.Cells. 2024 Aug 13;13(16):1338. doi: 10.3390/cells13161338. Cells. 2024. PMID: 39195228 Free PMC article. Review.
Cited by
-
The Autophagy Receptor SQSTM1/p62 Is a Restriction Factor of HCMV Infection.Viruses. 2024 Sep 10;16(9):1440. doi: 10.3390/v16091440. Viruses. 2024. PMID: 39339916 Free PMC article.
References
-
- Milbradt J., Hutterer C., Bahsi H., Wagner S., Sonntag E., Horn A.H., Kaufer B.B., Mori Y., Sticht H., Fossen T., et al. The Prolyl Isomerase Pin1 Promotes the Herpesvirus-Induced Phosphorylation-Dependent Disassembly of the Nuclear Lamina Required for Nucleocytoplasmic Egress. PLoS Pathog. 2016;12:e1005825. doi: 10.1371/journal.ppat.1005825. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

