Investigation of the Association between the Energy Metabolism of the Insect Vector Laodelphax striatellus and Rice Stripe Virus (RSV)
- PMID: 36298853
- PMCID: PMC9607531
- DOI: 10.3390/v14102298
Investigation of the Association between the Energy Metabolism of the Insect Vector Laodelphax striatellus and Rice Stripe Virus (RSV)
Abstract
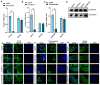
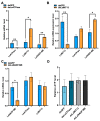
Viruses, as intracellular parasites, rely on the host organism to complete their life cycle. Although over 70% of plant viruses are transmitted by insect vectors, the role of vector energy metabolism on the infection process of insect-borne plant viruses is unclear. In this study, full-length cDNAs of three energy metabolism-related genes (LsATPase, LsMIT13 and LsNADP-ME) were obtained from the small brown planthopper (SBPH, Laodelphax striatellus), which transmits the Rice stripe virus (RSV). Expression levels of LsATPase, LsMIT13 and LsNADP-ME increased by 105%, 1120% and 259%, respectively, due to RSV infection. The repression of LsATPase, LsMIT13 or LsNADP-ME by RNAi had no effect on RSV nucleocapsid protein (NP) transcripts or protein levels. The repression of LsATPase caused a significant increase in LsMIT13 and LsNADP-ME transcript levels by 230% and 217%, respectively, and the repression of LsMIT13 caused a significant increase in LsNADP-ME mRNA levels. These results suggested that the silencing of LsATPase induced compensatory upregulation of LsMIT13 and LsNADP-ME, and silencing LsMIT13 induced compensatory upregulation of LsNADP-ME. Further study indicated that the co-silencing of LsATPase, LsMIT13 and LsNADP-ME in viruliferous SBPHs increased ATP production and RSV loads by 182% and 117%, respectively, as compared with nonviruliferous SBPHs. These findings indicate that SBPH energy metabolism is involved in RSV infection and provide insight into the association between plant viruses and energy metabolism in the insect vector.
Keywords: ATP synthase; Laodelphax striatellus; NAD-dependent malic enzyme; RSV; energy metabolism; mitochondrial import inner membrane translocases.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

