Involvement of endoplasmic reticulum stress and cell death by synthesized Pa-PDT in oral squamous cell carcinoma cells
- PMID: 36299346
- PMCID: PMC9588792
- DOI: 10.1016/j.jds.2022.02.006
Involvement of endoplasmic reticulum stress and cell death by synthesized Pa-PDT in oral squamous cell carcinoma cells
Abstract
Background/purpose: Photodynamic therapy (PDT) is a therapeutic alternative for malignant tumors that uses a photosensitizer. This study examined whether synthesized Pheophorbide a (Pa) -PDT induced apoptosis and autophagy involving endoplasmic reticulum (ER) stress in oral squamous cell carcinoma (OSCC) cells.
Materials and methods: Human OSCC cells were treated with Pa-PDT, and cell proliferation was examined by MTT assay. Apoptosis and autophagy were measured using Western blot analysis. ER stress was examined using RT-PCR and Western blot analysis. In vivo murine OSCC animal model were treated with intratumoral (IT) Pa-PDT, and investigated the therapeutic effect.
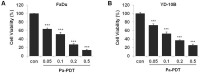
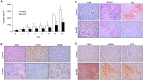
Results: Pa-PDT significantly inhibited the proliferation of human OSCC cells in a dose-dependent manner. Pa-PDT induced intrinsic apoptotic cell death and also induced autophagy. Pa-PDT induced ER stress which was observed as demonstrated by the up-regulation of the ER stress marker. Inhibition of the ER stress pathway using 4-phenylbutyric acid (PBA) decreased CHOP and induced inhibition of cell deaths. In addition, the inhibition of ER stress enhanced Pa-PDT mediated autophagy. IT Pa-PDT significantly inhibited the tumor growth and induced apoptosis, autophagy and ER stress in vivo OSCC cells transplanted model.
Conclusion: This study showed that synthesized Pa-PDT induced ER stress trigger apoptosis and apoptotic cell death pathways in OSCC cells. The inhibition of ER stress declined Pa-PDT mediated cytotoxicity with an increase of autophagy. These results may provide Pa-PDT exerts anti-tumor effects through ER stress pathway in OSCC cells and may provide a basis for developing Pa-PDT targeting ER stress as a therapy for OSCC.
Keywords: Apoptosis; Autophagy; ER stress; Oral sqaumous cell carcinoma; Pa-PDT.
© 2022 Association for Dental Sciences of the Republic of China. Publishing services by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest relevant to this article.
Figures




Similar articles
-
Synthesized Pheophorbide a-mediated photodynamic therapy induced apoptosis and autophagy in human oral squamous carcinoma cells.J Oral Pathol Med. 2013 Jan;42(1):17-25. doi: 10.1111/j.1600-0714.2012.01187.x. Epub 2012 Jun 28. J Oral Pathol Med. 2013. PMID: 22742535
-
Pheophorbide a-mediated photodynamic therapy induces apoptotic cell death in murine oral squamous cell carcinoma in vitro and in vivo.Oncol Rep. 2012 Jun;27(6):1772-8. doi: 10.3892/or.2012.1748. Epub 2012 Mar 27. Oncol Rep. 2012. PMID: 22470106
-
Intratumoral Photodynamic Therapy With Newly Synthesized Pheophorbide a in Murine Oral Cancer.Oncol Res. 2017 Jan 26;25(2):295-304. doi: 10.3727/096504016X14732527645922. Epub 2016 Sep 13. Oncol Res. 2017. PMID: 27629775 Free PMC article.
-
In Vitro Anti-tumor Effects of Photodynamic Therapy on Oral Squamous Cell Carcinoma: A Review.J Lasers Med Sci. 2022 Nov 20;13:e49. doi: 10.34172/jlms.2022.49. eCollection 2022. J Lasers Med Sci. 2022. PMID: 37041780 Free PMC article. Review.
-
Emerging Role of Autophagy in the Development and Progression of Oral Squamous Cell Carcinoma.Cancers (Basel). 2021 Dec 7;13(24):6152. doi: 10.3390/cancers13246152. Cancers (Basel). 2021. PMID: 34944772 Free PMC article. Review.
Cited by
-
Photodynamic Therapy for Eye, Ear, Laryngeal Area, and Nasal and Oral Cavity Diseases: A Review.Cancers (Basel). 2024 Feb 2;16(3):645. doi: 10.3390/cancers16030645. Cancers (Basel). 2024. PMID: 38339396 Free PMC article. Review.
-
Advancements in nanotechnology-driven photodynamic and photothermal therapies: mechanistic insights and synergistic approaches for cancer treatment.RSC Adv. 2024 Dec 10;14(52):38952-38995. doi: 10.1039/d4ra07114j. eCollection 2024 Dec 3. RSC Adv. 2024. PMID: 39659608 Free PMC article. Review.
-
Photodynamic Therapy: A Novel Approach for Head and Neck Cancer Treatment with Focusing on Oral Cavity.Biol Proced Online. 2024 Aug 17;26(1):25. doi: 10.1186/s12575-024-00252-3. Biol Proced Online. 2024. PMID: 39154015 Free PMC article. Review.
-
Activatable Photosensitizers: From Fundamental Principles to Advanced Designs.Angew Chem Int Ed Engl. 2025 Apr 7;64(15):e202423348. doi: 10.1002/anie.202423348. Epub 2025 Feb 21. Angew Chem Int Ed Engl. 2025. PMID: 39899458 Free PMC article. Review.
-
A bibliometric analysis of programmed cell death in oral cancer literature: research patterns and emerging trends (2000-2024).Discov Oncol. 2025 Apr 22;16(1):585. doi: 10.1007/s12672-025-02410-9. Discov Oncol. 2025. PMID: 40261469 Free PMC article.
References
-
- Karakullukcu B., Nyst H.J., van Veen R.L., et al. mTHPC mediated interstitial photodynamic therapy of recurrent nonmetastatic base of tongue cancers: development of a new method. Head Neck. 2012;34:1597–1606. - PubMed
-
- D'Cruz A.K., Robinson M.H., Biel M.A. mTHPC-mediated photodynamic therapy in patients with advanced, incurable head and neck cancer: a multicenter study of 128 patients. Head Neck. 2004;26:232–240. - PubMed
-
- Hopper C., Kübler A., Lewis H., Tan I.B., Putnam G. mTHPC mediated photodynamic therapy for early oral squamous cell carcinoma. Int J Cancer. 2004;111:138–146. - PubMed
-
- Leung W.N., Sun X., Mak N.K., Yow C.M. Photodynamic effects of mTHPC on human colon adenocarcinoma cells: photocytotoxicity, subcellular localization and apoptosis. Photochem Photobiol. 2002;75:406–411. - PubMed
-
- Paszko E., Ehrhardt C., Senge M.O., Kelleher D.P., Reynolds J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn Ther. 2011;8:14–29. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

