Impact of changes at the Candida albicans cell surface upon immunogenicity and colonisation in the gastrointestinal tract
- PMID: 36299406
- PMCID: PMC9589014
- DOI: 10.1016/j.tcsw.2022.100084
Impact of changes at the Candida albicans cell surface upon immunogenicity and colonisation in the gastrointestinal tract
Abstract
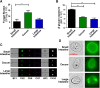
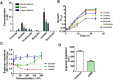
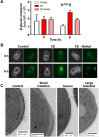
The immunogenicity of Candida albicans cells is influenced by changes in the exposure of microbe-associated molecular patterns (MAMPs) on the fungal cell surface. Previously, the degree of exposure on the C. albicans cell surface of the immunoinflammatory MAMP β-(1,3)-glucan was shown to correlate inversely with colonisation levels in the gastrointestinal (GI) tract. This is important because life-threatening systemic candidiasis in critically ill patients often arises from translocation of C. albicans strains present in the patient's GI tract. Therefore, using a murine model, we have examined the impact of gut-related factors upon β-glucan exposure and colonisation levels in the GI tract. The degree of β-glucan exposure was examined by imaging flow cytometry of C. albicans cells taken directly from GI compartments, and compared with colonisation levels. Fungal β-glucan exposure was lower in the cecum than the small intestine, and fungal burdens were correspondingly higher in the cecum. This inverse correlation did not hold for the large intestine. The gut fermentation acid, lactate, triggers β-glucan masking in vitro, leading to attenuated anti-Candida immune responses. Additional fermentation acids are present in the GI tract, including acetate, propionate, and butyrate. We show that these acids also influence β-glucan exposure on C. albicans cells in vitro and, like lactate, they influence β-glucan exposure via Gpr1/Gpa2-mediated signalling. Significantly, C. albicans gpr1Δ gpa2Δ cells displayed elevated β-glucan exposure in the large intestine and a corresponding decrease in fungal burden, consistent with the idea that Gpr1/Gpa2-mediated β-glucan masking influences colonisation of this GI compartment. Finally, extracts from the murine gut and culture supernatants from the mannan grazing gut anaerobe Bacteroides thetaiotaomicron promote β-glucan exposure at the C. albicans cell surface. Therefore, the local microbiota influences β-glucan exposure levels directly (via mannan grazing) and indirectly (via fermentation acids), whilst β-glucan masking appears to promote C. albicans colonisation of the murine large intestine.
Keywords: Candida albicans; Cell wall; Fungal immunogenicity; Gut colonisation; β-Glucan exposure.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Alessi A.M., Gray V., Farquharson F.M., Flores-López A., Shaw S., Stead D., et al. β-Glucan is a major growth substrate for human gut bacteria related to Coprococcus eutactus. Environ. Microbiol. 2020;22(6):2150–2164. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

