Involvement of ceramide biosynthesis in increased extracellular vesicle release in Pkd1 knock out cells
- PMID: 36299464
- PMCID: PMC9589111
- DOI: 10.3389/fendo.2022.1005639
Involvement of ceramide biosynthesis in increased extracellular vesicle release in Pkd1 knock out cells
Abstract
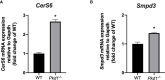
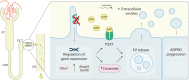
Autosomal Dominant Polycystic Kidney Disease (ADPKD) is an inherited disorder characterized by the development of renal cysts, which frequently leads to renal failure. Hypertension and other cardiovascular symptoms contribute to the high morbidity and mortality of the disease. ADPKD is caused by mutations in the PKD1 gene or, less frequently, in the PKD2 gene. The disease onset and progression are highly variable between patients, whereby the underlying mechanisms are not fully elucidated. Recently, a role of extracellular vesicles (EVs) in the progression of ADPKD has been postulated. However, the mechanisms stimulating EV release in ADPKD have not been addressed and the participation of the distal nephron segments is still uninvestigated. Here, we studied the effect of Pkd1 deficiency on EV release in wild type and Pkd1-/- mDCT15 and mIMCD3 cells as models of the distal convoluted tubule (DCT) and inner medullary collecting duct (IMCD), respectively. By using nanoparticle tracking analysis, we observed a significant increase in EV release in Pkd1-/- mDCT15 and mIMCD3 cells, with respect to the wild type cells. The molecular mechanisms leading to the changes in EV release were further investigated in mDCT15 cells through RNA sequencing and qPCR studies. Specifically, we assessed the relevance of purinergic signaling and ceramide biosynthesis enzymes. Pkd1-/- mDCT15 cells showed a clear upregulation of P2rx7 expression compared to wild type cells. Depletion of extracellular ATP by apyrase (ecto-nucleotidase) inhibited EV release only in wild type cells, suggesting an exacerbated signaling of the extracellular ATP/P2X7 pathway in Pkd1-/- cells. In addition, we identified a significant up-regulation of the ceramide biosynthesis enzymes CerS6 and Smpd3 in Pkd1-/- cells. Altogether, our findings suggest the involvement of the DCT in the EV-mediated ADPKD progression and points to the induction of ceramide biosynthesis as an underlying molecular mechanism. Further studies should be performed to investigate whether CerS6 and Smpd3 can be used as biomarkers of ADPKD onset, progression or severity.
Keywords: ADPKD; Autosomal Dominant Polycistic Kidney Disease; exosomes; extracellular ATP; extracellular vesicles; purinergic signaling.
Copyright © 2022 Carotti, van der Wijst, Verschuren, Rutten, Sommerdijk, Kaffa, Sommers, Rigalli and Hoenderop.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

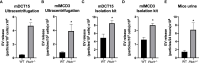
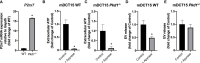
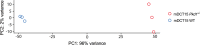
Similar articles
-
Extracellular vesicles contribute to early cyst development in autosomal dominant polycystic kidney disease by cell-to-cell communication.FASEB J. 2023 Jul;37(7):e23006. doi: 10.1096/fj.202300490R. FASEB J. 2023. PMID: 37249915
-
Polycystin-1 dysfunction impairs electrolyte and water handling in a renal precystic mouse model for ADPKD.Am J Physiol Renal Physiol. 2018 Sep 1;315(3):F537-F546. doi: 10.1152/ajprenal.00622.2017. Epub 2018 May 16. Am J Physiol Renal Physiol. 2018. PMID: 29767557
-
Loss of PKD1/polycystin-1 impairs lysosomal activity in a CAPN (calpain)-dependent manner.Autophagy. 2021 Sep;17(9):2384-2400. doi: 10.1080/15548627.2020.1826716. Epub 2020 Oct 6. Autophagy. 2021. PMID: 32967521 Free PMC article.
-
Cilia and polycystic kidney disease.Semin Cell Dev Biol. 2021 Feb;110:139-148. doi: 10.1016/j.semcdb.2020.05.003. Epub 2020 May 28. Semin Cell Dev Biol. 2021. PMID: 32475690 Review.
-
Translational research in ADPKD: lessons from animal models.Nat Rev Nephrol. 2014 Oct;10(10):587-601. doi: 10.1038/nrneph.2014.137. Epub 2014 Aug 19. Nat Rev Nephrol. 2014. PMID: 25137562 Review.
Cited by
-
Exploring the role of urinary extracellular vesicles in kidney physiology, aging, and disease progression.Am J Physiol Cell Physiol. 2023 Dec 1;325(6):C1439-C1450. doi: 10.1152/ajpcell.00349.2023. Epub 2023 Oct 16. Am J Physiol Cell Physiol. 2023. PMID: 37842748 Free PMC article. Review.
-
Translational regulation of PKD1 by evolutionarily conserved upstream open reading frames.RNA Biol. 2025 Dec;22(1):1-12. doi: 10.1080/15476286.2024.2448387. Epub 2025 Jan 5. RNA Biol. 2025. PMID: 39757590 Free PMC article. Review.
-
Microglial activation induces nitric oxide signalling and alters protein S-nitrosylation patterns in extracellular vesicles.J Extracell Vesicles. 2024 Jun;13(6):e12455. doi: 10.1002/jev2.12455. J Extracell Vesicles. 2024. PMID: 38887871 Free PMC article.
-
PKD1-mediated phosphorylation at dopamine D2 receptor serine 365 site in dorsal striatum underlies cocaine-induced locomotor hyperactivity.IBRO Neurosci Rep. 2025 Jun 19;19:124-132. doi: 10.1016/j.ibneur.2025.06.013. eCollection 2025 Dec. IBRO Neurosci Rep. 2025. PMID: 40606639 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

