Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination
- PMID: 36299530
- PMCID: PMC9588150
- DOI: 10.1093/ofid/ofac485
Long-term Protection Against Herpes Zoster by the Adjuvanted Recombinant Zoster Vaccine: Interim Efficacy, Immunogenicity, and Safety Results up to 10 Years After Initial Vaccination
Abstract
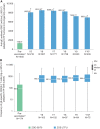
Approximately 10 years after vaccination with the recombinant zoster vaccine (RZV), an interim analysis of this follow-up study of the ZOE-50/70 trials demonstrated that efficacy against herpes zoster remained high. Moreover, the safety profile remained clinically acceptable, suggesting that the clinical benefit of the RZV in ≥50-year-olds is sustained up to 10 years.
Keywords: adjuvanted recombinant zoster vaccine; immune response persistence; long-term efficacy; long-term safety.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. K.A.S. is a Modis employee, working on behalf of the GSK group of companies. A.M.O., M.S., A.S., and P.P. are employees of the GSK group of companies. P.P. and A.S. hold shares/stocks in the GSK group of companies as part of their employee remuneration. J.D.D. reports grants from the GSK group of companies, Sanofi, and MSD; consulting fees from Sanofi Pasteur and MSD paid to him and his institution; honoraria from the GSK group of companies and SEQIRUS (to him and his institution), outside the submitted work. J.C.T. reports grant/research support from the GSK group of companies. The authors have no other financial or nonfinancial interests to declare. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
-
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352:2271–84. - PubMed
-
- Lal H, Cunningham AL, Godeaux O, et al. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N Engl J Med 2015; 372:2087–96. - PubMed
-
- Cunningham AL, Lal H, Kovac M, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med 2016; 375:1019–32. - PubMed

