Naphthalimide-phenothiazine dyads: effect of conformational flexibility and matching of the energy of the charge-transfer state and the localized triplet excited state on the thermally activated delayed fluorescence
- PMID: 36300011
- PMCID: PMC9577389
- DOI: 10.3762/bjoc.18.149
Naphthalimide-phenothiazine dyads: effect of conformational flexibility and matching of the energy of the charge-transfer state and the localized triplet excited state on the thermally activated delayed fluorescence
Abstract
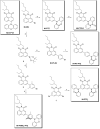
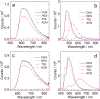
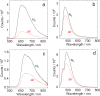
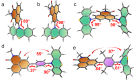
In order to investigate the joint influence of the conformation flexibility and the matching of the energies of the charge-transfer (CT) and the localized triplet excited (3LE) states on the thermally activated delayed fluorescence (TADF) in electron donor-acceptor molecules, a series of compact electron donor-acceptor dyads and a triad were prepared, with naphthalimide (NI) as electron acceptor and phenothiazine (PTZ) as electron donor. The NI and PTZ moieties are either directly connected at the 3-position of NI and the N-position of the PTZ moiety via a C-N single bond, or they are linked through a phenyl group. The tuning of the energy order of the CT and LE states is achieved by oxidation of the PTZ unit into the corresponding sulfoxide, whereas conformation restriction is imposed by introducing ortho-methyl substituents on the phenyl linker, so that the coupling magnitude between the CT and the 3LE states can be controlled. The singlet oxygen quantum yield (ΦΔ) of NI-PTZ is moderate in n-hexane (HEX, ΦΔ = 19%). TADF was observed for the dyads, the biexponential luminescence lifetime are 16.0 ns (99.9%)/14.4 μs (0.1%) for the dyad and 7.2 ns (99.6%)/2.0 μs (0.4%) for the triad. Triplet state was observed in the nanosecond transient absorption spectra with lifetimes in the 4-48 μs range. Computational investigations show that the orthogonal electron donor-acceptor molecular structure is beneficial for TADF. These calculations indicate small energetic difference between the 3LE and 3CT states, which are helpful for interpreting the ns-TA spectra and the origins of TADF in NI-PTZ, which is ultimately due to the small energetic difference between the 3LE and 3CT states. Conversely, NI-PTZ-O, which has a higher CT state and bears a much more stabilized 3LE state, does not show TADF.
Keywords: TADF; charge-transfer; electron donor; intersystem crossing; triplet state.
Copyright © 2022, Ye et al.
Figures







References
-
- Cai X, Su S-J. Adv Funct Mater. 2018;28:1802558. doi: 10.1002/adfm.201802558. - DOI
-
- Cao X, Zhang D, Zhang S, Tao Y, Huang W. J Mater Chem C. 2017;5:7699–7714. doi: 10.1039/c7tc02481a. - DOI
-
- Im Y, Byun S Y, Kim J H, Lee D R, Oh C S, Yook K S, Lee J Y. Adv Funct Mater. 2017;27:1603007. doi: 10.1002/adfm.201603007. - DOI
-
- Zhang W, Song H, Kong J, Kuang Z, Li M, Guo Q, Chen C-f, Xia A. J Phys Chem C. 2019;123:19322–19332. doi: 10.1021/acs.jpcc.9b03867. - DOI
-
- Fukuzumi S. Pure Appl Chem. 2007;79:981–991. doi: 10.1351/pac200779060981. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
