Enhanced thermal and photo-stability of a para-substituted dicumyl ketone intercalated in a layered double hydroxide
- PMID: 36300029
- PMCID: PMC9588950
- DOI: 10.3389/fchem.2022.1004586
Enhanced thermal and photo-stability of a para-substituted dicumyl ketone intercalated in a layered double hydroxide
Abstract
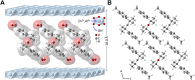
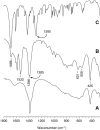
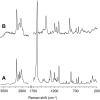
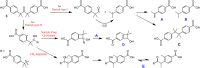
A ketodiacid, 4,4'-dicarboxylate-dicumyl ketone (3), has been intercalated into a Zn, Al layered double hydroxide (LDH) by a coprecipitation synthesis strategy. The structure and chemical composition of the resultant hybrid material (LDH-KDA3) were characterized by powder X-ray diffraction (PXRD), FT-IR, FT-Raman and solid-state 13C{1H} NMR spectroscopies, scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), thermogravimetric analysis (TGA), and elemental analysis (CHN). PXRD showed that the dicarboxylate guest molecules assembled into a monolayer to give a basal spacing of 18.0 Å. TGA revealed that the organic guest starts to decompose at a significantly higher temperature (ca. 330°C) than that determined for the free ketodiacid (ca. 230°C). Photochemical experiments were performed to probe the photoreactivity of the ketoacid in the crystalline state, in solution, and as a guest embedded within the photochemically-inert LDH host. Irradiation of the bulk crystalline ketoacid results in photodecarbonylation and the exclusive formation of the radical-radical combination product. Solution studies employing the standard myoglobin (Mb) assay for quantification of released CO showed that the ketoacid behaved as a photoactivatable CO-releasing molecule for transfer of CO to heme proteins, although the photoreactivity was low. No photoinduced release of CO was found for the LDH system, indicating that molecular confinement enhanced the photo-stability of the hexasubstituted ketone. To better understand the behavior of 3 under irradiation, a more comprehensive study, involving excitation of this compound in DMSO-d6 followed by 1H NMR, UV-Vis and fluorescence spectroscopy, was undertaken and further rationalized with the help of time-dependent density functional theory (TDDFT) electronic quantum calculations. The photophysical study showed the formation of a less emissive compound (or compounds). New signals in the 1H NMR spectra were attributed to photoproducts obtained via Norrish type I α-cleavage decarbonylation and Norrish type II (followed by CH3 migration) pathways. TDDFT calculations predicted that the formation of a keto-enol system (via a CH3 migration step in the type II pathway) was highly favorable and consistent with the observed spectral data.
Keywords: CO-releasing molecules; TDDFT calculations; hexasubstituted ketones; intercalation; ketodiacid; layered double hydroxides; myoglobin assay; photodecarbonylation.
Copyright © 2022 Costa, Monteiro, Nunes Barradas, Ferreira, Cunha, Gomes, Gonçalves, Seixas de Melo and Pillinger.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Akanksha,, Maiti D. (2012). Microwave-assisted palladium mediated decarbonylation reaction: Synthesis of eulatachromene. Green Chem. 14, 2314–2320. 10.1039/c2gc35622h - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

