Acridine yellow G (AYG) as a photo-induced electron transfer (PET) photocatalyst employed for the radical Michael-Mannich cyclocondensation of imines
- PMID: 36300032
- PMCID: PMC9590109
- DOI: 10.3389/fchem.2022.1015330
Acridine yellow G (AYG) as a photo-induced electron transfer (PET) photocatalyst employed for the radical Michael-Mannich cyclocondensation of imines
Abstract
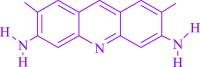
A four-component domino Michael-Mannich cyclocondensation of amines, dialkyl acetylenedicarboxylaes, and formaldehyde was utilized to develop a green technique for sans metal combination of polyfunctionalized dihydro-2-oxypyrroles. It involves visible light as an environmentally friendly power source and acridine yellow G (AYG) as a photo-induced electron transfer (PET) photocatalyst. The motivation behind this examination was to expand the utilization of a non-metal dye that is both reasonable and broadly accessible. Photochemically catalyzed AYG flaunts exceptional returns, energy effectiveness, and natural agreeableness, as well as extraordinary iota economy, efficient highlights, and comfort of purpose. Key abilities consist of an easy experimental setup, big substrate tolerance, finance-friendly, clean painting-up strategies within the absence of tedious separation techniques, and minimized the quantity of waste for each organic transformation. The type of yields is pretty uniform (85-97%, average 92.09%), and the shape of reaction times might be very speedy (15-30 min, average 21.59 min), and the factor stated inside the dialogue is that the method tolerates quite a number electron-donating and electron-withdrawing functional groups, while, however, giving extremely good yields. The response within the reason is insensitive to the person of the substituents. Subsequently, many compounds and natural factors can be followed over the course of time. Shockingly, gram-scale cyclization is conceivable, proposing that the strategy could be utilized in industry.
Keywords: acridine yellow G (AYG); photo-induced electron transfer (PET); photochemical synthesis; polyfunctionalized dihydro-2-oxypyrroles; renewable energy source.
Copyright © 2022 Mohamadpour.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Amat A. M., Arques A., Galindo F., Miranda M. A., Santos-Juanes L., Vercher R. F., et al. (2007). Acridine yellow as solar photocatalyst for enhancing biodegradability and eliminating ferulic acid as model pollutant. Appl. Catal. B Environ. 73, 220–226. 10.1016/j.apcatb.2006.12.003 - DOI
-
- Arques A., Amat A. M., Santos-Juanes L., Vercher R. F., Marin M. L., Miranda M. A. (2009). Abatement of methidathion and carbaryl from aqueous solutions using organic photocatalysts. Catal. Today 144, 106–111. 10.1016/j.cattod.2008.11.013 - DOI
-
- Borthwick A. D., Crame A. J., Ertl P. F., Exall A. M., Haley T. M., Hart G. J., et al. (2002). Design and synthesis of pyrrolidine-5, 5-trans-lactams (5-oxohexahydropyrrolo [3, 2-b] pyrroles) as novel mechanism-based inhibitors of human cytomegalovirus protease. 2. Potency and chirality. J. Med. Chem. 45, 1–18. 10.1021/jm0102203 - DOI - PubMed
LinkOut - more resources
Full Text Sources

