Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy
- PMID: 36300110
- PMCID: PMC9589261
- DOI: 10.3389/fimmu.2022.1018903
Regulation of autophagy fires up the cold tumor microenvironment to improve cancer immunotherapy
Abstract
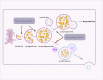
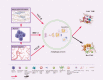
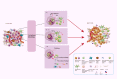
Immunotherapies, such as immune checkpoint inhibitors (ICIs) and chimeric antigen receptor (CAR) T cells, have revolutionized the treatment of patients with advanced and metastatic tumors resistant to traditional therapies. However, the immunosuppressed tumor microenvironment (TME) results in a weak response to immunotherapy. Therefore, to realize the full potential of immunotherapy and obstacle barriers, it is essential to explore how to convert cold TME to hot TME. Autophagy is a crucial cellular process that preserves cellular stability in the cellular components of the TME, contributing to the characterization of the immunosuppressive TME. Targeted autophagy ignites immunosuppressive TME by influencing antigen release, antigen presentation, antigen recognition, and immune cell trafficking, thereby enhancing the effectiveness of cancer immunotherapy and overcoming resistance to immunotherapy. In this review, we summarize the characteristics and components of TME, explore the mechanisms and functions of autophagy in the characterization and regulation of TME, and discuss autophagy-based therapies as adjuvant enhancers of immunotherapy to improve the effectiveness of immunotherapy.
Keywords: antigen presentation; autophagy; immune cells; immunogenic cell death; immunotherapy; tumor microenvironment.
Copyright © 2022 Jin, Sun, Wang, Zhou, Yang and Zhou.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. . Low-dose radiotherapy reverses tumor immune desertification and resistance to Immunotherapy. Cancer Discov (2022) 12(1):108–33. doi: 10.1158/2159-8290.Cd-21-0003 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

