The role of mitochondria in the pathogenesis of Kawasaki disease
- PMID: 36300112
- PMCID: PMC9592088
- DOI: 10.3389/fimmu.2022.1017401
The role of mitochondria in the pathogenesis of Kawasaki disease
Abstract
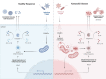
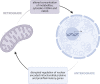
Kawasaki disease is a systemic vasculitis, especially of the coronary arteries, affecting children. Despite extensive research, much is still unknown about the principal driver behind the amplified inflammatory response. We propose mitochondria may play a critical role. Mitochondria serve as a central hub, influencing energy generation, cell proliferation, and bioenergetics. Regulation of these biological processes, however, comes at a price. Release of mitochondrial DNA into the cytoplasm acts as damage-associated molecular patterns, initiating the development of inflammation. As a source of reactive oxygen species, they facilitate activation of the NLRP3 inflammasome. Kawasaki disease involves many of these inflammatory pathways. Progressive mitochondrial dysfunction alters the activity of immune cells and may play a role in the pathogenesis of Kawasaki disease. Because they contain their own genome, mitochondria are susceptible to mutation which can propagate their dysfunction and immunostimulatory potential. Population-specific variants in mitochondrial DNA have also been linked to racial disparities in disease risk and treatment response. Our objective is to critically examine the current literature of mitochondria's role in coordinating proinflammatory signaling pathways, focusing on potential mitochondrial dysfunction in Kawasaki disease. No association between impaired mitochondrial function and Kawasaki disease exists, but we suggest a relationship between the two. We hypothesize a framework of mitochondrial determinants that may contribute to ethnic/racial disparities in the progression of Kawasaki disease.
Keywords: Kawasaki disease; inflammasome; inflammation; mitochondria; mitochondrial DNA; mitophagy; reactive oxygen species.
Copyright © 2022 Beckley, Shrestha, Singh and Portman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- McCrindle BW, Rowley AH, Newburger JW, Burns J.C., Bolger AF, Gewitz M, et al. . Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American heart association. Circulation (2017) 135(17):e927–99. doi: 10.1161/CIR.0000000000000484 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

