Efficacy and safety profile of phosphodiesterase 4 inhibitor in the treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials
- PMID: 36300119
- PMCID: PMC9589065
- DOI: 10.3389/fimmu.2022.1021537
Efficacy and safety profile of phosphodiesterase 4 inhibitor in the treatment of psoriasis: A systematic review and meta-analysis of randomized controlled trials
Abstract
Background: Systemic therapy is an important treatment for psoriasis. Phosphodiesterase 4 (PDE4) inhibitors are new candidates for psoriasis therapy.
Objectives: To evaluate the efficacy and safety of PDE4 inhibitors in psoriasis.
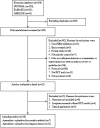
Method: Randomized clinical trials with PDE4 inhibitors vs placebos in patients with psoriasis were identified from MEDLINE, Embase, Cochrane Controlled Register of Trials, ClinicalTrials.gov, from inception to July 14, 2022. The study was registered in PROSPERO (CRD42022345700).
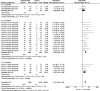
Results: 18 studies were identified, 9 of which included moderate-to-severe plaque psoriasis, 2 mild-to-moderate plaque psoriasis, and 7 psoriatic arthritis. A total of 6036 patients were included. Only one oral PDE4 inhibitor, apremilast, met the inclusion criteria. Overall, compared with the placebo, apremilast was associated with higher response rates in PASI-75 (RR, 3.22; 95% CI, 2.59-4.01), ScPGA of 0 or 1 (RR, 2.21; 95% CI, 1.69-2.91), PPPGA of 0 or 1 (RR 2.33; 95%CI, 1.16-4.66), and a significant decrease in NPASI (SMD, -0.46; 95% CI, -0.58 to -0.33). There were no significant differences in serious adverse events. Subgroup analyses showed that significantly more patients achieved PASI-75 after 16 weeks of therapy with apremilast of 20 mg bid (RR, 2.82; 95% CI, 2.01-3.95) and 30 mg bid (RR, 4.08; 95% CI, 3.12-5.33). Heterogeneity was not significant across studies.
Conclusion: Apremilast is a safe and effective treatment for plaque psoriasis and psoriatic arthritis, especially for difficult-to-treat sites.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier (CRD42022345700).
Keywords: PDE4 inhibitor; apremilast; meta-analysis; psoriasis; treatment strategies.
Copyright © 2022 Kang, Chen and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Rigopoulos D, Baran R, Chiheb S, Daniel CR, 3rd, Di Chiacchio N, Gregoriou S. Recommendations for the definition, evaluation, and treatment of nail psoriasis in adult patients with no or mild skin psoriasis: A dermatologist and nail expert group consensus. J Am Acad Dermatol (2019) 81(1):228–40. doi: 10.1016/j.jaad.2019.01.072 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous