SOT101 induces NK cell cytotoxicity and potentiates antibody-dependent cell cytotoxicity and anti-tumor activity
- PMID: 36300122
- PMCID: PMC9590108
- DOI: 10.3389/fimmu.2022.989895
SOT101 induces NK cell cytotoxicity and potentiates antibody-dependent cell cytotoxicity and anti-tumor activity
Abstract
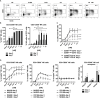
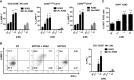
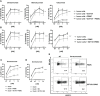
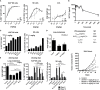
SOT101 is a superagonist fusion protein of interleukin (IL)-15 and the IL-15 receptor α (IL-15Rα) sushi+ domain, representing a promising clinical candidate for the treatment of cancer. SOT101 among other immune cells specifically stimulates natural killer (NK) cells and memory CD8+ T cells with no significant expansion or activation of the regulatory T cell compartment. In this study, we showed that SOT101 induced expression of cytotoxic receptors NKp30, DNAM-1 and NKG2D on human NK cells. SOT101 stimulated dose-dependent proliferation and the relative expansion of both major subsets of human NK cells, CD56brightCD16- and CD56dimCD16+, and these displayed an enhanced cytotoxicity in vitro. Using human PBMCs and isolated NK cells, we showed that SOT101 added concomitantly or used for immune cell pre-stimulation potentiated clinically approved monoclonal antibodies Cetuximab, Daratumumab and Obinutuzumab in killing of tumor cells in vitro. The anti-tumor efficacy of SOT101 in combination with Daratumumab was assessed in a solid multiple myeloma xenograft in CB17 SCID mouse model testing several combination schedules of administration in the early and late therapeutic setting of established tumors in vivo. SOT101 and Daratumumab monotherapies decreased with various efficacy tumor growth in vivo in dependence on the advancement of the tumor development. The combination of both drugs showed the strongest anti-tumor efficacy. Specifically, the sequencing of both drugs did not matter in the early therapeutic setting where a complete tumor regression was observed in all animals. In the late therapeutic treatment of established tumors Daratumumab followed by SOT101 administration or a concomitant administration of both drugs showed a significant anti-tumor efficacy over the respective monotherapies. These results suggest that SOT101 might significantly augment the anti-tumor activity of therapeutic antibodies by increasing NK cell-mediated activity in patients. These results support the evaluation of SOT101 in combination with Daratumumab in clinical studies and present a rationale for an optimal clinical dosing schedule selection.
Keywords: NK cells; RLI-15; antibody-dependent cytotoxicity; immunotherapy; interleukin-15; therapeutic antibodies.
Copyright © 2022 Antosova, Podzimkova, Tomala, Augustynkova, Sajnerova, Nedvedova, Sirova, de Martynoff, Bechard, Moebius, Kovar, Spisek and Adkins.
Conflict of interest statement
Authors ZA, NP, KA, KS, EN, UM, RS, and IA were employed by SOTIO Biotech a.s. Authors GM and DB were employed by Cytune Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

