Therapeutic function of a novel rat induced pluripotent stem cell line in a 6‑OHDA‑induced rat model of Parkinson's disease
- PMID: 36300203
- PMCID: PMC9652010
- DOI: 10.3892/ijmm.2022.5196
Therapeutic function of a novel rat induced pluripotent stem cell line in a 6‑OHDA‑induced rat model of Parkinson's disease
Abstract
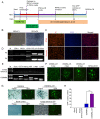
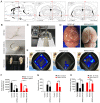
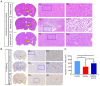
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder of the central nervous system that results from the loss of dopaminergic (DA) nigral neurons. Induced pluripotent stem cells (iPSCs) have shown potential for cell transplantation treatment of neurodegenerative disorders. In the present study, the small molecules CHIR99021 and RepSox (CR) significantly facilitated reprogramming and enhanced the efficiency of GFP+/iPS‑like colonies [rat iPSCs induced by OCT3/4, Sox2, Klf4, c‑Myc, Nanog and Lin28 + CR (RiPSCs‑6F/CR)] generation by ~4.0‑fold during lentivirus‑mediated reprogramming of six transcription factors in rat embryonic fibroblasts. The generation of iPSCs was detected by reverse transcription‑PCR, immunofluorescence and western blot analysis. Subsequently, RiPSCs‑6F/CR were stereotactically transplanted into the right medial forebrain bundle (MFB) of 6‑hydroxydopamine‑lesioned rats with PD. The transplanted RiPSCs‑6F/CR survived and functioned in the MFB of rats with PD for ≥20 weeks, and significantly improved functional restoration from their PD‑related behavioral defects. Furthermore, the grafted RiPSCs‑6F/CR could migrate and differentiate into various neurocytes in vivo, including γ aminobutyric acid‑ergic, DA neurons and glial cells. In conclusion, the present study confirmed that RiPSCs‑6F/CR induced by small molecules could be used as potential donor material for neural grafting to remodel basal ganglia circuitry in neurodegenerative diseases.
Keywords: Parkinson's disease; cell reprogramming; neural transplantation; rat induced pluripotent stem cells; small molecules.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



Similar articles
-
Miniature-swine iPSC-derived GABA progenitor cells function in a rat Parkinson's disease model.Cell Tissue Res. 2023 Mar;391(3):425-440. doi: 10.1007/s00441-022-03736-4. Epub 2023 Jan 16. Cell Tissue Res. 2023. PMID: 36645476
-
[Directed differentiation of human induced pluripotent stem cells into midbrain].Nan Fang Yi Ke Da Xue Xue Bao. 2023 Feb 20;43(2):175-182. doi: 10.12122/j.issn.1673-4254.2023.02.03. Nan Fang Yi Ke Da Xue Xue Bao. 2023. PMID: 36946035 Free PMC article. Chinese.
-
Human induced pluripotent stem cell-derived neurons improve motor asymmetry in a 6-hydroxydopamine-induced rat model of Parkinson's disease.Cytotherapy. 2015 May;17(5):665-79. doi: 10.1016/j.jcyt.2015.02.001. Epub 2015 Mar 5. Cytotherapy. 2015. PMID: 25747741
-
Potential application of induced pluripotent stem cells in cell replacement therapy for Parkinson's disease.CNS Neurol Disord Drug Targets. 2011 Jun;10(4):449-58. doi: 10.2174/187152711795563994. CNS Neurol Disord Drug Targets. 2011. PMID: 21495962 Review.
-
[Cell replacement therapy for Parkinson's disease using iPS cells].Rinsho Shinkeigaku. 2013;53(11):1009-12. doi: 10.5692/clinicalneurol.53.1009. Rinsho Shinkeigaku. 2013. PMID: 24291862 Review. Japanese.
Cited by
-
Genetically engineered BMSCs promote dopamine secretion and ameliorate motor dysfunction in a Parkinson's disease rat model.Sci Rep. 2025 Apr 11;15(1):12514. doi: 10.1038/s41598-025-97557-4. Sci Rep. 2025. PMID: 40217082 Free PMC article.
-
[Parkin deletion affects PINK1/Parkin-mediated mitochondrial autophagy to exacerbate neuroinflammation and accelerate progression of Parkinson's disease in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Dec 20;44(12):2359-2366. doi: 10.12122/j.issn.1673-4254.2024.12.11. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39725624 Free PMC article. Chinese.
-
[Rho kinase inhibitor Y27632 promotes survival of human induced pluripotent stem cells during differentiation into functional midbrain dopaminergic progenitor cells in vitro].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Feb 20;44(2):236-243. doi: 10.12122/j.issn.1673-4254.2024.02.05. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 38501408 Free PMC article. Chinese.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

