Four-Component Strain-Release-Driven Synthesis of Functionalized Azetidines
- PMID: 36300572
- PMCID: PMC10099845
- DOI: 10.1002/anie.202214049
Four-Component Strain-Release-Driven Synthesis of Functionalized Azetidines
Abstract
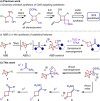
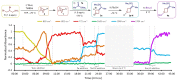
Despite the favorable properties that azetidine rings can engender on drug-compounds, methods for the diversity-oriented synthesis of azetidine-based structures are significantly underdeveloped. Herein, we report the successful realization of a multicomponent [1,2]-Brook rearrangement/strain-release-driven anion relay sequence and its application to the modular synthesis of substituted azetidines. The rapidity of the reaction, as confirmed by in situ infra-red spectroscopy, leverages the strain-release ring-opening of azabicyclo[1.1.0]butane to drive the equilibrium of the Brook rearrangement. The three electrophilic coupling partners, added sequentially to azabicyclo[1.1.0]butyl-lithium, could be individually varied to access a diverse compound library. The utility of this methodology was demonstrated in a 4-step synthesis of the EP2 receptor antagonist PF-04418948.
Keywords: Azetidines; Bicyclic Compounds; Heterocycles; Multicomponent Reaction; Strained Molecules.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

