Effect of regulating macrophage polarization phenotype on intervertebral disc degeneration
- PMID: 36301028
- PMCID: PMC9609449
- DOI: 10.1002/iid3.714
Effect of regulating macrophage polarization phenotype on intervertebral disc degeneration
Abstract
Background: Macrophages are the only inflammatory cells that can penetrate the closed nucleus pulposus and their polarization plays an important role in intervertebral disc degeneration (IVDD). This paper attempted to investigate the pathogenesis of IVDD by altering the polarization state of macrophages.
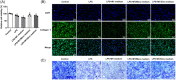
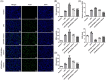
Methods: Macrophage RAW264.7 cells were induced by interferonγ (IFN-γ) and lipopolysaccharide (LPS). The polarization of RAW264.7 cells was estimated by western blot and immunofluorescence. The expressions of inflammatory factors were detected by ELISA. Subsequently, RAW264.7 cells were treated with different concentrations of minocycline (Mino) and sinomenine (Sino), followed by the assessment of cell viability with cell counting kit-8 kit. Then, RAW264.7 cell culture medium was collected for the culture of human nucleus pulposus cells (NPCs). Toluidine blue staining and type II collagen staining were applied to assay the level of type II collagen. The cell apoptosis, oxidative stress, and nitric oxide (NO) level were appraised by TUNEL, oxidative stress kits and NO kit, respectively. Western blot was employed to test the levels of apoptosis- and oxidative stress-related proteins.
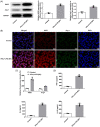
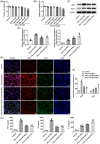
Results: IFN-γ and LPS could induce M1 polarization of RAW264.7 cells. Mino and Sino could reduce the polarization of RAW264.7 cells toward M1. M1-polarized medium inhibited LPS-induced activity, inflammation, and damage of NPCs, which were enhanced by Mino and Sino in medium.
Conclusion: M1 polarization of macrophages promoted LPS-induced inflammation and damage of NPCs.
Keywords: M1 polarization; inflammation; intervertebral disc degeneration; macrophages.
© 2022 The Authors. Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Cannata F, Vadalà G, Ambrosio L, et al. Intervertebral disc degeneration: a focus on obesity and type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3224. - PubMed
-
- Navone SE, Marfia G, Giannoni A, et al Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 2017;32:523‐542. - PubMed
-
- Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL‐1 beta and TNF‐alpha in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

